Use of RNAlater in fluorescence-activated cell sorting (FACS) reduces the fluorescence from GFP but not from DsRed
- PMID: 21134271
- PMCID: PMC3017069
- DOI: 10.1186/1756-0500-3-328
Use of RNAlater in fluorescence-activated cell sorting (FACS) reduces the fluorescence from GFP but not from DsRed
Abstract
Background: Flow cytometry utilizes signals from fluorescent markers to separate targeted cell populations for gene expression studies. However, the stress of the FACS process could change normal gene expression profiles. RNAlater could be used to stop such changes in original gene expression profiles through its ability to denature RNase and other proteins. The normal conformational structure of fluorescent proteins must be maintained in order to fluoresce. Whether or not RNAlater would affect signals from different types of intrinsic fluorescent proteins is crucial to its use in flow cytometry; this question has not been investigated in detail.
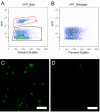
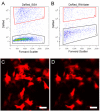
Findings: To address this question, we analyzed the effect of RNAlater on fluorescence intensity of GFP, YFP, DsRed and small fluorescent molecules attached to secondary antibodies (Cy2 and Texas-Red) when used in flow cytometry. FACS results were confirmed with fluorescence microscopy. Our results showed that exposure of YFP and GFP containing cells to RNAlater reduces the intensity of their fluorescence to such an extent that separation of such labeled cells is difficult if not impossible. In contrast, signals from DsRed2, Cy2 and Texas-Red were not affected by RNAlater treatment. In addition, the background fluorescence and clumping of dissociated cells are altered by RNAlater treatment.
Conclusions: When considering gene expression studies using cell sorting with RNAlater, DsRed is the fluorescent protein of choice while GFP/YFP have severe limitations because of their reduced fluorescence. It is necessary to examine the effects of RNAlater on signals from fluorescent markers and the physical properties (e.g., clumping) of the cells before considering its use in cell sorting.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources

