Early eradication of persistent Salmonella infection primes antibody-mediated protective immunity to recurrent infection
- PMID: 21134485
- PMCID: PMC3056909
- DOI: 10.1016/j.micinf.2010.11.004
Early eradication of persistent Salmonella infection primes antibody-mediated protective immunity to recurrent infection
Abstract
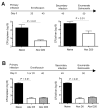
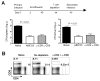
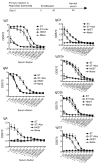
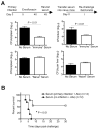
Typhoid fever is a systemic, persistent infection caused by host-specific strains of Salmonella. Although the use of antibiotics has reduced the complications associated with primary infection, recurrent infection remains an important cause of ongoing human morbidity and mortality. Herein, we investigated the impacts of antibiotic eradication of primary infection on protection against secondary recurrent infection. Using a murine model of persistent Salmonella infection, we demonstrate protection against recurrent infection is sustained despite early eradication of primary infection. In this model, protection is not mediated by CD4(+) or CD8(+) T cells because depletion of these cells either alone or in combination prior to rechallenge does not abrogate protection. Instead, infection followed by antibiotic-mediated clearance primes robust levels of Salmonella-specific antibody that can adoptively transfer protection to naïve mice. Thus, eradication of persistent Salmonella infection primes antibody-mediated protective immunity to recurrent infection.
Copyright © 2010 Institut Pasteur. Published by Elsevier SAS. All rights reserved.
Figures
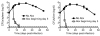

References
-
- Yew FS, Chew SK, Goh KT, Monteiro EH, Lim YS. Typhoid fever in Singapore: a review of 370 cases. J Trop Med Hyg. 1991;94:352–357. - PubMed
-
- Islam A, Butler T, Ward LR. Reinfection with a different Vi-phage type of Salmonella typhi in an endemic area. The Journal of infectious diseases. 1987;155:155–156. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

