Synthesis of a-factor peptide from Saccharomyces cerevisiae and photoactive analogues via Fmoc solid phase methodology
- PMID: 21134758
- PMCID: PMC3037452
- DOI: 10.1016/j.bmc.2010.11.006
Synthesis of a-factor peptide from Saccharomyces cerevisiae and photoactive analogues via Fmoc solid phase methodology
Abstract
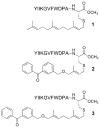
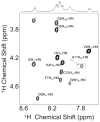
a-Factor from Saccharomyces cerevisiae is a farnesylated dodecapeptide involved in mating. The molecule binds to a G-protein coupled receptor and hence serves as a simple system for studying the interactions between prenylated molecules and their cognate receptors. Here, we describe the preparation of a-factor and two photoactive analogues via Fmoc solid-phase peptide synthesis using hydrazinobenzoyl AM NovaGel™ resin; the structure of the synthetic a-factor was confirmed by MS-MS analysis and NMR; the structures of the analogues were confirmed by MS-MS analysis. Using a yeast growth arrest assay, the analogues were found to have activity comparable to a-factor itself.
Copyright © 2010 Elsevier Ltd. All rights reserved.
Figures
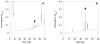
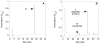





References
-
- Kurjan J. Ann Rev Biochem. 1992;61:1097. - PubMed
-
- Anderegg RJ, Betz R, Carr SA, Crabb JW, Duntze W. J Biol Chem. 1988;263:18236. - PubMed
-
- Naider F, Estephan R, Englander J, Suresh Babu VV, Arevalo E, Samples K, Becker Jeffrey M. Biopolymers. 2004;76:119. - PubMed
-
- Zhang FL, Casey PJ. Annu Rev Biochem. 1996;65:241. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

