Native properdin binds to Chlamydia pneumoniae and promotes complement activation
- PMID: 21134964
- PMCID: PMC3028849
- DOI: 10.1128/IAI.00980-10
Native properdin binds to Chlamydia pneumoniae and promotes complement activation
Abstract
Activation of complement represents one means of natural resistance to infection from a wide variety of potential pathogens. Recently, properdin, a positive regulator of the alternative pathway of complement, has been shown to bind to surfaces and promote complement activation. Here we studied whether properdin-mediated complement activation occurs on the surface of Chlamydia pneumoniae, an obligate intracellular Gram-negative bacterium that causes 10 to 20% of community-acquired pneumonia. We have determined for the first time that the physiological P₂, P₃, and P₄ forms of human properdin bind to the surface of Chlamydia pneumoniae directly. The binding of these physiological forms accelerates complement activation on the Chlamydia pneumoniae surface, as measured by C3b and C9 deposition. Finally, properdin-depleted serum could not control Chlamydia pneumoniae infection of HEp-2 cells compared with normal human serum. However, after addition of native properdin, the properdin-depleted serum recovered the ability to control the infection. Altogether, our data suggest that properdin is a pattern recognition molecule that plays a role in resistance to Chlamydia infection.
Figures


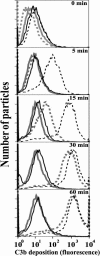

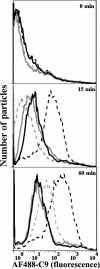

References
-
- Belland, R., D. M. Ojcius, and G. I. Byrne. 2004. Chlamydia. Nat. Rev. Microbiol. 2:530-531. - PubMed
-
- Belland, R. J., S. P. Ouellette, J. Gieffers, and G. I. Byrne. 2004. Chlamydia pneumoniae and atherosclerosis. Cell. Microbiol. 6:117-127. - PubMed
-
- Bertsche, A., M. H. Wagner, R. Bollmann, M. Obladen, and U. Felderhoff-Mueser. 2008. An unusual manifestation of a neonatal Chlamydia infection. J. Child Neurol. 23:948-949. - PubMed
-
- Biesecker, G., and H. J. Muller-Eberhard. 1980. The ninth component of human complement: purification and physicochemical characterization. J. Immunol. 124:1291-1296. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

