Mouse peptidoglycan recognition protein PGLYRP-1 plays a role in the host innate immune response against Listeria monocytogenes infection
- PMID: 21134971
- PMCID: PMC3028829
- DOI: 10.1128/IAI.00466-10
Mouse peptidoglycan recognition protein PGLYRP-1 plays a role in the host innate immune response against Listeria monocytogenes infection
Abstract
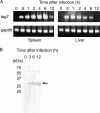
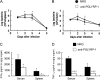
The role of mouse peptidoglycan recognition protein PGLYRP-1 in innate immunity against Listeria monocytogenes infection was studied. The recombinant mouse PGLYRP-1 and a polyclonal antibody specific to PGLYRP-1 were prepared. The mouse PGLYRP-1 showed antibacterial activities against L. monocytogenes and other Gram-positive bacteria. PGLYRP-1 mRNA expression was induced in the spleens and livers of mice infected with L. monocytogenes. The viable bacterial number increased, and the production of cytokines such as gamma interferon (IFN-γ) and tumor necrosis factor alpha (TNF-α) was reduced in mice when mice had been injected with anti-PGLYRP-1 antibody before infection. The levels of IFN-γ and TNF-α titers in the organs were higher and the viable bacterial number was reduced in mice injected with recombinant mouse PGLYRP-1 (rmPGLYRP-1) before infection. PGLYRP-1 could directly induce these cytokines in spleen cell cultures. The elimination of intracellular bacteria was upregulated in NMuLi hepatocyte cells overexpressing PGLYRP-1. The enhancement of the elimination of L. monocytogenes from the organs was observed in IFN-γ(-/-) mice by rmPGLYRP-1 administration but not in TNF-α(-/-) mice. These results suggest that PGLYRP-1 plays a role in innate immunity against L. monocytogenes infection by inducing TNF-α.
Figures






References
-
- Bischoff, V., et al. 2004. Function of the drosophila pattern-recognition receptor PGRP-SD in the detection of Gram-positive bacteria. Nat. Immunol. 5:1175-1180. - PubMed
-
- Carpenter, S., and L. A. O'Neill. 2007. How important are Toll-like receptors for antimicrobial responses? Cell. Microbiol. 9:1891-1901. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

