Stopping smokeless tobacco with varenicline: randomised double blind placebo controlled trial
- PMID: 21134997
- PMCID: PMC2997603
- DOI: 10.1136/bmj.c6549
Stopping smokeless tobacco with varenicline: randomised double blind placebo controlled trial
Abstract
Objective: To assess the efficacy and safety of varenicline (a licensed cigarette smoking cessation aid) in helping users of smokeless tobacco to quit.
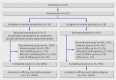
Design: Double blind, placebo controlled, parallel group, multicentre, randomised controlled trial.
Setting: Medical clinics (mostly primary care) in Norway and Sweden.
Participants: Men and women aged ≥18 who used smokeless tobacco at least eight times a day, with no abstinence period over three months within one year before screening, who wanted to quit all tobacco use. Participants were excluded if they used any other form of tobacco (except smokeless tobacco) or medication to stop smoking within three months of screening or had any pre-existing medical or psychiatric condition.
Interventions: Varenicline 1 mg twice daily (titrated during the first week) or placebo for 12 weeks, with 14 weeks' follow-up after treatment.
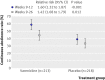
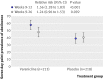
Main outcome measures: The primary end point was the four week continuous abstinence rate at the end of treatment (weeks 9-12) confirmed with cotinine concentration. A secondary end point was continuous abstinence rate for weeks 9-26. Safety and tolerability were also evaluated.
Results: 431 participants (213 varenicline; 218 placebo) were randomised and received at least one dose of study drug. Participants' demographics and baseline use of smokeless tobacco were similar (89% (189) and 90% (196), respectively, were men; mean age in both groups was 43.9; participants used smokeless tobacco products about 15 times a day, and about 80% first used smokeless tobacco within 30 minutes after awakening). Continuous abstinence rate at week 9-12 was higher in the varenicline group than the placebo group (59% (125) v 39% (85); relative risk 1.60, 95% confidence interval 1.32 to 1.87, P<0.001; risk difference 20%; number needed to treat 5). The advantage of varenicline over placebo persisted through 14 weeks of follow-up (continuous abstinence rate at week 9-26 was 45% (95) v 34% (73); relative risk 1.42, 1.08 to 1.79, P=0.012; risk difference 11%; number needed to treat 9). The most common adverse events in the varenicline group compared with the placebo group were nausea (35% (74) v 6% (14)), fatigue (10% (22) v 7% (15)), headache (10% (22) v 9% (20)), and sleep disorder (10% (22) v 7% (15)). Few adverse events led to discontinuation of treatment (9% (19) and 4% (9), respectively), and serious adverse events occurred in two (1%) and three (1%) participants, respectively.
Conclusion: Varenicline can help people to give up smokeless tobacco and has an acceptable safety profile. The response rate in the placebo group in this study was high, suggesting a population less resistant to treatment than smokers.
Trial registration: NCT00717093.
Conflict of interest statement
Competing interests: All authors have completed the Unified Competing Interest form at
Figures
Comment in
-
Drug treatment for users of smokeless tobacco.BMJ. 2010 Dec 6;341:c6598. doi: 10.1136/bmj.c6598. BMJ. 2010. PMID: 21134998 No abstract available.
References
-
- Ministry of Health and Social Affairs in Sweden and Swedish National Institute of Public Health. Results from the National Health Interview Survey. Health on equal terms, 2008—living habits. Ministry of Health and Social Affairs in Sweden and Swedish National Institute of Public Health, 2008.
-
- Fiore MC, Jaén CR, Baker TB, Bailey WC, Benowitz NL, Curry SJ, et al. Clinical practice guideline. Treating tobacco use and dependence: 2008 update. US Department of Health and Human Services: Public Health Service, 2008.
-
- Lund KE, Tefre EM, Amundsen A, Nordlund S. [Cigarette smoking, use of snuff and other risk behaviour among students.] Tidsskr Nor Laegeforen 2008;128:1808-11. - PubMed
-
- Centers for Disease Control and Prevention. Use of smokeless tobacco among adults—United States, 1991. MMWR Morb Mortal Wkly Rep 1993;42:263-6. - PubMed
-
- Centers for Disease Control and Prevention. Smoking and tobacco use: national youth tobacco survey (NYTS), 2006. NYTS Data and Documentation, 2006.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
