A microbial rhodopsin with a unique retinal composition shows both sensory rhodopsin II and bacteriorhodopsin-like properties
- PMID: 21135094
- PMCID: PMC3057805
- DOI: 10.1074/jbc.M110.190058
A microbial rhodopsin with a unique retinal composition shows both sensory rhodopsin II and bacteriorhodopsin-like properties
Abstract
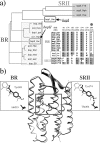
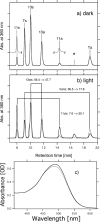
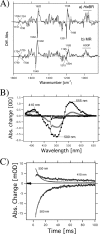
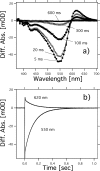
Rhodopsins possess retinal chromophore surrounded by seven transmembrane α-helices, are widespread in prokaryotes and in eukaryotes, and can be utilized as optogenetic tools. Although rhodopsins work as distinctly different photoreceptors in various organisms, they can be roughly divided according to their two basic functions, light-energy conversion and light-signal transduction. In microbes, light-driven proton transporters functioning as light-energy converters have been modified by evolution to produce sensory receptors that relay signals to transducer proteins to control motility. In this study, we cloned and characterized two newly identified microbial rhodopsins from Haloquadratum walsbyi. One of them has photochemical properties and a proton pumping activity similar to the well known proton pump bacteriorhodopsin (BR). The other, named middle rhodopsin (MR), is evolutionarily transitional between BR and the phototactic sensory rhodopsin II (SRII), having an SRII-like absorption maximum, a BR-like photocycle, and a unique retinal composition. The wild-type MR does not have a light-induced proton pumping activity. On the other hand, a mutant MR with two key hydrogen-bonding residues located at the interaction surface with the transducer protein HtrII shows robust phototaxis responses similar to SRII, indicating that MR is potentially capable of the signaling. These results demonstrate that color tuning and insertion of the critical threonine residue occurred early in the evolution of sensory rhodopsins. MR may be a missing link in the evolution from type 1 rhodopsins (microorganisms) to type 2 rhodopsins (animals), because it is the first microbial rhodopsin known to have 11-cis-retinal similar to type 2 rhodopsins.
Figures


References
-
- Spudich J. L. (2006) Trends Microbiol. 14, 480–487 - PubMed
-
- Zhang F., Aravanis A. M., Adamantidis A., de Lecea L., Deisseroth K. (2007) Nat. Rev. Neurosci. 8, 577–581 - PubMed
-
- Ruiz-González M. X., Marín I. (2004) J. Mol. Evol. 58, 348–358 - PubMed
-
- Sharma A. K., Spudich J. L., Doolittle W. F. (2006) Trends Microbiol. 14, 463–469 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

