A high redox potential form of cytochrome c550 in photosystem II from Thermosynechococcus elongatus
- PMID: 21135104
- PMCID: PMC3057795
- DOI: 10.1074/jbc.M110.170126
A high redox potential form of cytochrome c550 in photosystem II from Thermosynechococcus elongatus
Abstract
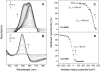
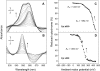
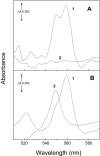
Cytochrome c(550) (cyt c(550)) is a component of photosystem II (PSII) from cyanobacteria, red algae, and some other eukaryotic algae. Its physiological role remains unclear. In the present work, measurements of the midpoint redox potential (E(m)) were performed using intact PSII core complexes preparations from a histidine-tagged PSII mutant strain of the thermophilic cyanobacterium Thermosynechococcus (T.) elongatus. When redox titrations were done in the absence of redox mediators, an E(m) value of +200 mV was obtained for cyt c(550). This value is ∼300 mV more positive than that previously measured in the presence of mediators (E(m) = -80 mV). The shift from the high potential form (E(m) = +200 mV) to the low potential form (E(m) = -80 mV) of cyt c(550) is attributed to conformational changes, triggered by the reduction of a component of PSII that is sequestered and out of equilibrium with the medium, most likely the Mn(4)Ca cluster. This reduction can occur when reduced low potential redox mediators are present or under highly reducing conditions even in the absence of mediators. Based on these observations, it is suggested that the E(m) of +200 mV obtained without mediators could be the physiological redox potential of the cyt c(550) in PSII. This value opens the possibility of a redox function for cyt c(550) in PSII.
Figures




References
-
- Barber J. (2009) Chem. Soc. Rev. 38, 185–196 - PubMed
-
- Zouni A., Witt H. T., Kern J., Fromme P., Krauss N., Saenger W., Orth P. (2001) Nature 409, 739–743 - PubMed
-
- Ferreira K. N., Iverson T. M., Maghlaoui K., Barber J., Iwata S. (2004) Science 303, 1831–1838 - PubMed
-
- Biesiadka J., Loll B., Kern J., Irrgang K. D., Zouni A. (2004) Phys. Chem. Chem. Phys. 6, 4733–4736

