The disintegrin-like and cysteine-rich domains of ADAM-9 mediate interactions between melanoma cells and fibroblasts
- PMID: 21135106
- PMCID: PMC3057808
- DOI: 10.1074/jbc.M110.168617
The disintegrin-like and cysteine-rich domains of ADAM-9 mediate interactions between melanoma cells and fibroblasts
Abstract
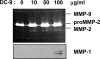
A characteristic of malignant cells is their capacity to invade their surrounding and to metastasize to distant organs. During these processes, proteolytic activities of tumor and stromal cells modify the extracellular matrix to produce a microenvironment suitable for their growth and migration. In recent years the family of ADAM proteases has been ascribed important roles in these processes. ADAM-9 is expressed in human melanoma at the tumor-stroma border where direct or indirect interactions between tumor cells and fibroblasts occur. To analyze the role of ADAM-9 for the interaction between melanoma cells and stromal fibroblasts, we produced the recombinant disintegrin-like and cysteine-rich domain of ADAM-9 (DC-9). Melanoma cells and human fibroblasts adhered to immobilized DC-9 in a Mn(2+)-dependent fashion suggesting an integrin-mediated process. Inhibition studies showed that adhesion of fibroblasts was mediated by several β1 integrin receptors independent of the RGD and ECD recognition motif. Furthermore, interaction of fibroblasts and high invasive melanoma cells with soluble recombinant DC-9 resulted in enhanced expression of MMP-1 and MMP-2. Silencing of ADAM-9 in melanoma cells significantly reduced cell adhesion to fibroblasts. Ablation of ADAM-9 in fibroblasts almost completely abolished these cellular interactions and melanoma cell invasion in vitro. In summary, these results suggest that ADAM-9 expression plays an important role in mediating cell-cell contacts between fibroblasts and melanoma cells and that these interactions contribute to proteolytic activities required during invasion of melanoma cells.
Figures
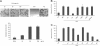
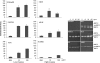
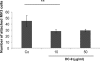
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

