PTP1B regulates Eph receptor function and trafficking
- PMID: 21135139
- PMCID: PMC3002030
- DOI: 10.1083/jcb.201005035
PTP1B regulates Eph receptor function and trafficking
Abstract
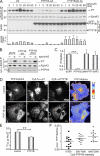
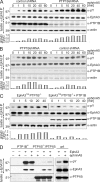
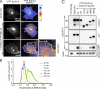
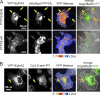
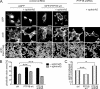
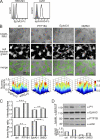
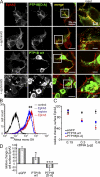
Eph receptors orchestrate cell positioning during normal and oncogenic development. Their function is spatially and temporally controlled by protein tyrosine phosphatases (PTPs), but the underlying mechanisms are unclear and the identity of most regulatory PTPs are unknown. We demonstrate here that PTP1B governs signaling and biological activity of EphA3. Changes in PTP1B expression significantly affect duration and amplitude of EphA3 phosphorylation and biological function, whereas confocal fluorescence lifetime imaging microscopy (FLIM) reveals direct interactions between PTP1B and EphA3 before ligand-stimulated receptor internalization and, subsequently, on endosomes. Moreover, overexpression of wild-type (w/t) PTP1B and the [D-A] substrate-trapping mutant decelerate ephrin-induced EphA3 trafficking in a dose-dependent manner, which reveals its role in controlling EphA3 cell surface concentration. Furthermore, we provide evidence that in areas of Eph/ephrin-mediated cell-cell contacts, the EphA3-PTP1B interaction can occur directly at the plasma membrane. Our studies for the first time provide molecular, mechanistic, and functional insights into the role of PTP1B controlling Eph/ephrin-facilitated cellular interactions.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

