CDK5RAP2 stimulates microtubule nucleation by the gamma-tubulin ring complex
- PMID: 21135143
- PMCID: PMC3002024
- DOI: 10.1083/jcb.201007030
CDK5RAP2 stimulates microtubule nucleation by the gamma-tubulin ring complex
Abstract
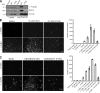
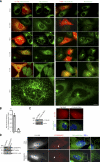
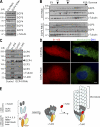
CDK5RAP2 is a human microcephaly protein that contains a γ-tubulin complex (γ-TuC)-binding domain conserved in Drosophila melanogaster centrosomin and Schizosaccharomyces pombe Mto1p and Pcp1p, which are γ-TuC-tethering proteins. In this study, we show that this domain within CDK5RAP2 associates with the γ-tubulin ring complex (γ-TuRC) to stimulate its microtubule-nucleating activity and is therefore referred to as the γ-TuRC-mediated nucleation activator (γ-TuNA). γ-TuNA but not its γ-TuC-binding-deficient mutant stimulates microtubule nucleation by purified γ-TuRC in vitro and induces extensive, γ-TuRC-dependent nucleation of microtubules in a microtubule regrowth assay. γ-TuRC bound to γ-TuNA contains NME7, FAM128A/B, and actin in addition to γ-tubulin and GCP2-6. RNA interference-mediated depletion of CDK5RAP2 impairs both centrosomal and acentrosomal microtubule nucleation, although γ-TuRC assembly is unaffected. Collectively, these results suggest that the γ-TuNA found in CDK5RAP2 has regulatory functions in γ-TuRC-mediated microtubule nucleation.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

