Seamless phase I-II trial design for assessing toxicity and efficacy for targeted agents
- PMID: 21135145
- PMCID: PMC4391513
- DOI: 10.1158/1078-0432.CCR-10-1262
Seamless phase I-II trial design for assessing toxicity and efficacy for targeted agents
Abstract
Purpose: The premise for phase I trials for cytostatic agents is different from that of cytotoxic agents. For cytostatic agents, toxicity and efficacy do not necessarily increase monotonically with increasing dose levels, but likely plateau after they reach maximal toxicity or efficacy. Here, we propose a phase I-II trial design to assess both toxicity and efficacy to find the best dose as well as a good dose.
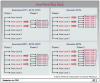
Experimental design: We propose a 2-step dose-finding trial for assessing both toxicity and efficacy for a targeted agent. The 1st step uses a traditional phase I trial design. This step only assesses toxicity and finds the maximal tolerated dose (MTD). For the 2nd step, we propose a modified phase II selection design for 2 or 3 dose levels at and below the MTD to determine efficacy and evaluate each dose level by both efficacy and toxicity.
Results and conclusion: Simulation studies are done on several combinations of toxicity and efficacy scenarios to assess the operating statistics of our proposed trial design. We then compare our results with a traditional phase I trial followed by a single-arm phase II trial using the same total sample size. The proposed design does better in most cases than a traditional design using the same overall sample size. This design allows assessing a few dose levels more closely for both efficacy and toxicity and provides greater certainty of having correctly determined the best dose level before launching into a large efficacy trial.
©2010 AACR.
Conflict of interest statement
No potential conflicts of interest were disclosed.
Figures


References
-
- Storer BE. Choosing a phase I design. In: Crowley J, Power Ankerst D, editors. Handbook of statistics in clinical oncology. 2. Boca Raton (FL): CRC Press; 2006. pp. 59–75.
-
- Thall PF, Cook JD. Dose-finding based on efficacy-toxicity trade offs. Biometrics. 2004;60:684–93. - PubMed
-
- Zhang W, Sargent DJ, Mandrekar S. An adaptive dose-finding design incorporating both toxicity and efficacy. Stat Med. 2006;25:2365–83. - PubMed
-
- Hunsberger S, Rubinstein LV, Dancey J, Korn EL, et al. Dose escalation trial designs based on a molecularly targeted endpoint. Stat Med. 2005;24:2171–81. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

