Gene expression profile correlates with T-cell infiltration and relative survival in glioblastoma patients vaccinated with dendritic cell immunotherapy
- PMID: 21135147
- PMCID: PMC3071163
- DOI: 10.1158/1078-0432.CCR-10-2563
Gene expression profile correlates with T-cell infiltration and relative survival in glioblastoma patients vaccinated with dendritic cell immunotherapy
Abstract
Purpose: To assess the feasibility, safety, and toxicity of autologous tumor lysate-pulsed dendritic cell (DC) vaccination and toll-like receptor (TLR) agonists in patients with newly diagnosed and recurrent glioblastoma. Clinical and immune responses were monitored and correlated with tumor gene expression profiles.
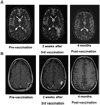
Experimental design: Twenty-three patients with glioblastoma (WHO grade IV) were enrolled in this dose-escalation study and received three biweekly injections of glioma lysate-pulsed DCs followed by booster vaccinations with either imiquimod or poly-ICLC adjuvant every 3 months until tumor progression. Gene expression profiling, immunohistochemistry, FACS, and cytokine bead arrays were performed on patient tumors and peripheral blood mononuclear cells.
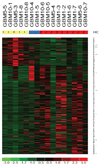
Results: DC vaccinations are safe and not associated with any dose-limiting toxicity. The median overall survival from the time of initial surgical diagnosis of glioblastoma was 31.4 months, with a 1-, 2-, and 3-year survival rate of 91%, 55%, and 47%, respectively. Patients whose tumors had mesenchymal gene expression signatures exhibited increased survival following DC vaccination compared with historic controls of the same genetic subtype. Tumor samples with a mesenchymal gene expression signature had a higher number of CD3(+) and CD8(+) tumor-infiltrating lymphocytes compared with glioblastomas of other gene expression signatures (P = 0.006).
Conclusion: Autologous tumor lysate-pulsed DC vaccination in conjunction with TLR agonists is safe as adjuvant therapy in newly diagnosed and recurrent glioblastoma patients. Our results suggest that the mesenchymal gene expression profile may identify an immunogenic subgroup of glioblastoma that may be more responsive to immune-based therapies.
©2010 AACR.
Conflict of interest statement
Figures



References
-
- Deorah S, Lynch CF, Sibenaller ZA, Ryken TC. Trends in brain cancer incidence and survival in the United States: Surveillance, Epidemiology, and End Results Program, 1973 to 2001. Neurosurg Focus. 2006;20:E1. - PubMed
-
- Cohen MH, Li Shen Y, Keegan P, Pazdur R. FDA Drug Approval Summary: Bevacizumab (Avastin(R)) as Treatment of Recurrent Glioblastoma Multiforme. Oncologist. 2009 - PubMed
-
- Lai A, Filka E, McGibbon B, et al. Phase II Pilot Study of Bevacizumab in Combination With Temozolomide and Regional Radiation Therapy for Up-Front Treatment of Patients With Newly Diagnosed Glioblastoma Multiforme: Interim Analysis of Safety and Tolerability. Int J Radiat Oncol Biol Phys. 2008 - PubMed
-
- Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. - PubMed
-
- Yang MY, Zetler PM, Prins RM, Khan-Farooqi H, Liau LM. Immunotherapy for patients with malignant glioma: from theoretical principles to clinical applications. Expert Rev Neurother. 2006;6:1481–1494. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

