Alloantibodies prevent the induction of transplantation tolerance by enhancing alloreactive T cell priming
- PMID: 21135169
- PMCID: PMC8351406
- DOI: 10.4049/jimmunol.1001172
Alloantibodies prevent the induction of transplantation tolerance by enhancing alloreactive T cell priming
Abstract
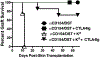
Circulating alloantibodies in transplant recipients are often associated with increased Ab-mediated as well as cellular rejection. We tested the hypothesis that alloantibodies facilitate cellular rejection by functioning as opsonins to enhance T cell activation using a BALB/c to C57BL/6 heart or skin transplant model. Long-term heart and skin survival induced with anti-CD154 alone or in combination with donor-specific transfusion (DST), respectively, was abrogated by the presence of anti-K(d) mAbs, and alloreactive T cell activation as well as acute rejection was observed. The prevention of graft acceptance in the skin model was dependent on anti-K(d) binding to and converting DST from tolerigenic to immunogenic. Adoptive transfer of CFSE-labeled TCR-transgenic T cells into B6 recipients treated with anti-CD154/DST revealed the ability of anti-K(d) to enhance the proliferation of anti-K(d)-specific T cells via the indirect pathway as well as of non-K(d)-reactive, recipient MHC-restricted CD4(+) and CD8(+) T cells. Thus, alloantibodies with restricted specificity are able to facilitate the indirect presentation as well as the cross-presentation of a larger repertoire of "linked" donor-derived Ags. These observations highlight the ability of alloantibodies to function not only in classical humoral rejection but also as opsonins that facilitate the CD40-CD154-independent activation of alloreactive T cells.
Conflict of interest statement
Figures
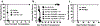
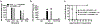
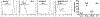
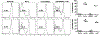
References
-
- Moxham VF, Karegli J, Phillips RE, Brown KL, Tapmeier TT, Hangartner R, Sacks SH, and Wong W. 2008. Homeostatic proliferation of lymphocytes results in augmented memory-like function and accelerated allograft rejection. J Immunol 180:3910–3918. - PubMed
-
- Chen Y, Heeger PS, and Valujskikh A. 2004. In vivo helper functions of alloreactive memory CD4+ T cells remain intact despite donor-specific transfusion and anti-CD40 ligand therapy. J Immunol 172:5456–5466. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

