Critical modifier role of membrane-cystic fibrosis transmembrane conductance regulator-dependent ceramide signaling in lung injury and emphysema
- PMID: 21135173
- PMCID: PMC3119853
- DOI: 10.4049/jimmunol.1002850
Critical modifier role of membrane-cystic fibrosis transmembrane conductance regulator-dependent ceramide signaling in lung injury and emphysema
Abstract
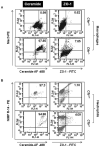
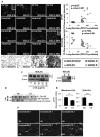
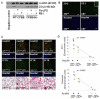
Ceramide accumulation mediates the pathogenesis of chronic obstructive lung diseases. Although an association between lack of cystic fibrosis transmembrane conductance regulator (CFTR) and ceramide accumulation has been described, it is unclear how membrane-CFTR may modulate ceramide signaling in lung injury and emphysema. Cftr(+/+) and Cftr(-/-) mice and cells were used to evaluate the CFTR-dependent ceramide signaling in lung injury. Lung tissue from control and chronic obstructive pulmonary disease patients was used to verify the role of CFTR-dependent ceramide signaling in pathogenesis of chronic emphysema. Our data reveal that CFTR expression inversely correlates with severity of emphysema and ceramide accumulation in chronic obstructive pulmonary disease subjects compared with control subjects. We found that chemical inhibition of de novo ceramide synthesis controls Pseudomonas aeruginosa-LPS-induced lung injury in Cftr(+/+) mice, whereas its efficacy was significantly lower in Cftr(-/-) mice, indicating that membrane-CFTR is required for controlling lipid-raft ceramide levels. Inhibition of membrane-ceramide release showed enhanced protective effect in controlling P. aeruginosa-LPS-induced lung injury in Cftr(-/-) mice compared with that in Cftr(+/+) mice, confirming our observation that CFTR regulates lipid-raft ceramide levels and signaling. Our results indicate that inhibition of de novo ceramide synthesis may be effective in disease states with low CFTR expression like emphysema and chronic lung injury but not in complete absence of lipid-raft CFTR as in ΔF508-cystic fibrosis. In contrast, inhibiting membrane-ceramide release has the potential of a more effective drug candidate for ΔF508-cystic fibrosis but may not be effectual in treating lung injury and emphysema. Our data demonstrate the critical role of membrane-localized CFTR in regulating ceramide accumulation and inflammatory signaling in lung injury and emphysema.
Figures




References
-
- Wedzicha JA, Donaldson GC. Exacerbations of chronic obstructive pulmonary disease. Respir Care. 2003;48:1204–1213. discussion 1213-1205. - PubMed
-
- Heijerman H. Infection and inflammation in cystic fibrosis: a short review. J Cyst Fibros. 2005;4(Suppl 2):3–5. - PubMed
-
- Martin RJ. Infections and asthma. Clin Chest Med. 2006;27:87–98. vi. - PubMed
-
- Murray TS, Egan M, Kazmierczak BI. Pseudomonas aeruginosa chronic colonization in cystic fibrosis patients. Curr Opin Pediatr. 2007;19:83–88. - PubMed
-
- Murphy TF, Brauer AL, Eschberger K, Lobbins P, Grove L, Cai X, Sethi S. Pseudomonas aeruginosa in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2008;177:853–860. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

