Alkylated porphyrins have broad antiviral activity against hepadnaviruses, flaviviruses, filoviruses, and arenaviruses
- PMID: 21135183
- PMCID: PMC3028764
- DOI: 10.1128/AAC.00989-10
Alkylated porphyrins have broad antiviral activity against hepadnaviruses, flaviviruses, filoviruses, and arenaviruses
Abstract
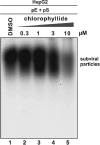
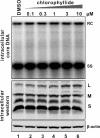
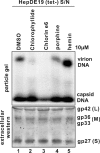
We screened ∼2,200 compounds known to be safe in people for the ability to reduce the amount of virion-associated hepatitis B virus (HBV) DNA in the culture medium of producer cells. These efforts led to the discovery of an alkylated porphyrin, chlorophyllide, as the compound that achieved the greatest reduction in signal. Here we report that chlorophyllide directly and quantitatively disrupted HBV virions at micromolar concentrations, resulting in the loss of all detectable virion DNA, without detectably affecting cell viability or intracellular viral gene products. Chemophores of chlorophyllide were also tested. Chlorin e6, a metal-free chlorophyllide-like molecule, showed the strongest antiviral activity against HBV as well as profound antiviral effects on other enveloped viruses, such as hepatitis C virus (HCV), human immunodeficiency virus (HIV), dengue virus (DENV), Marburg virus (MARV), Tacaribe virus (TCRV), and Junin viruses (JUNV). Remarkably, chlorin e6 inactivated DENV at subnanomolar-level concentrations. However, the compound had no antiviral effect against encephalomyocarditis virus and adenovirus, suggesting that chlorin e6 may be less active or inactive against nonenveloped viruses. Although other porphyrin derivatives have been previously reported to possess antiviral activity, this is the first analysis of the biochemical impact of chlorophyllide and chlorin e6 against HBV and of the dramatic anti-infectivity impact upon DENV. The possible application of this family of compounds as antiviral agents, as microbicides and systemic virus neutralizing agents, is discussed.
Figures




References
-
- Bleicher, K. H., H. J. Bohm, K. Muller, and A. I. Alanine. 2003. Hit and lead generation: beyond high-throughput screening. Nat. Rev. Drug Discov. 2:369-378. - PubMed
-
- Bremer, C. M., C. Bung, N. Kott, M. Hardt, and D. Glebe. 2009. Hepatitis B virus infection is dependent on cholesterol in the viral envelope. Cell. Microbiol. 11:249-260. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

