Phase II study of bevacizumab plus temozolomide during and after radiation therapy for patients with newly diagnosed glioblastoma multiforme
- PMID: 21135282
- PMCID: PMC3058273
- DOI: 10.1200/JCO.2010.30.2729
Phase II study of bevacizumab plus temozolomide during and after radiation therapy for patients with newly diagnosed glioblastoma multiforme
Abstract
Purpose: This open-label, prospective, multicenter single-arm phase II study combined bevacizumab (BV) with radiation therapy (RT) and temozolomide (TMZ) for the treatment of newly diagnosed glioblastoma (GBM). The objectives were to determine the efficacy of this treatment combination and the associated toxicity.
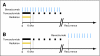
Patients and methods: Seventy patients with newly diagnosed GBM were enrolled between August 2006 and November 2008. Patients received standard RT starting within 3 to 6 weeks after surgery with concurrent administration of daily TMZ and biweekly BV. After completion of RT, patients resumed TMZ for 5 days every 4 weeks and continued biweekly BV. MGMT promoter methylation was assessed on patient tumor tissue. A University of California, Los Angeles/Kaiser Permanente Los Angeles (KPLA) control cohort of newly diagnosed patients treated with first-line RT and TMZ who had mostly received BV at recurrence was derived for comparison.
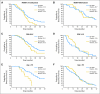
Results: The overall survival (OS) and progression-free survival (PFS) were 19.6 and 13.6 months, respectively, compared to 21.1 and 7.6 months in the University of California, Los Angeles/KPLA control cohort, and 14.6 and 6.9 months in the European Organisation for Research and Treatment of Cancer-National Cancer Institute of Canada cohort. Correlation of MGMT promoter methylation and improved OS and PFS was retained in the study group. Comparative subset analysis showed that poor prognosis patients (recursive partitioning analysis class V/VI) may derive an early benefit from the use of first-line BV. Toxicity attributable to RT/TMZ was similar, and additional toxicities were consistent with those reported in other BV trials.
Conclusion: Patients treated with BV and TMZ during and after RT showed improved PFS without improved OS compared to the University of California, Los Angeles/KPLA control group. Additional studies are warranted to determine if BV administered first-line improves survival compared to BV at recurrence.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures



Comment in
-
Taming glioblastoma by targeting angiogenesis: 3 years later.J Clin Oncol. 2011 Jan 10;29(2):124-6. doi: 10.1200/JCO.2010.32.5282. Epub 2010 Dec 6. J Clin Oncol. 2011. PMID: 21135277 No abstract available.
References
-
- Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. The Lancet Oncology. 2009;10:459–466. - PubMed
-
- Curran WJ, Jr, Scott CB, Horton J, et al. Recursive partitioning analysis of prognostic factors in three Radiation Therapy Oncology Group malignant glioma trials. J Natl Cancer Inst. 1993;85:704–710. - PubMed
-
- Friedman HS, Prados MD, Wen PY, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27:4733–4740. - PubMed
-
- Chen HX, Cleck JN. Adverse effects of anticancer agents that target the VEGF pathway. Nat Rev Clin Oncol. 2009;6:465–477. - PubMed
-
- Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

