A Reactivity-Driven Approach to the Discovery and Development of Gold-Catalyzed Organic Reactions
- PMID: 21135915
- PMCID: PMC2996726
- DOI: 10.1055/s-0029-1219369
A Reactivity-Driven Approach to the Discovery and Development of Gold-Catalyzed Organic Reactions
Abstract
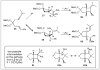
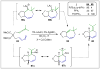
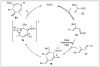
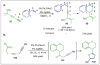
Approaches to research in organic chemistry are as numerous as the reactions they describe. In this account, we describe our reactivity-based approach. Using our work in the area of gold-catalysis as a background, we discuss how a focus on reaction mechanism and reactivity paradigms can lead to the rapid discovery of new synthetic tools.
Figures


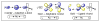
























References
-
- Lewis GN. Science. 1909;30:1. - PubMed
-
-
For recent discussions relevant to this approach see: Nicolaou KC, Snyder SA. Proc Natl Acad Sci USA. 2004;101:11929.Mohr JT, Krout MR, Stoltz BM. Nature. 2008;455:323.Shenvi RA, O’Malley DP, Baran PS. Acc Chem Res. 2009;42:530.Wender PA, Verma VA, Paxton TJ, Pillow TH. Acc Chem Res. 2008;41:40.Young IS, Baran PS. Nature Chem. 2009;1:193.Morten CJ, Byers JA, Van Dyke AR, Vilotijevic I, Jamison TF. Chem Soc Rev. 2009;38:3175.
-
-
- Grubbs RH, Chang S. Tetrahedron. 1998;54:4413.
- Dick AR, Sanford MS. Tetrahedron. 2006;62:2439.
- Hartwig JF. Nature. 2008;455:314. - PMC - PubMed
- Buchwald SL. Acc Chem Res. 2008;41:1439. - PubMed
- Sherry BD, Fürstner A. Acc Chem Res. 2008;41:1500. - PubMed
- Fu GC. Acc Chem Res. 2008;41:1555. - PMC - PubMed
- Afagh NA, Yudin AK. Angew Chem, Int Ed. 2009 doi: 10.1002/anie.200901317. - DOI - PubMed
-
- Francis MB, Jamison TF, Jacobsen EN. Curr Opin Chem Biol. 1998;2:422. - PubMed
- Weber L, Illgen K, Almstetter M. Synlett. 1999:366.
- Kanan MW, Rozenman MM, Sakurai K, Snyder TM, Liu DR. Nature. 2004;431:545. - PMC - PubMed
- Beeler AB, Su S, Singleton CA, Porco JA., Jr J Am Chem Soc. 2007;129:1413. - PubMed
- Rozenman MM, Kanan MW, Liu DR. J Am Chem Soc. 2007;129:14933. - PMC - PubMed
- Gorin DJ, Kamlet AS, Liu DR. J Am Chem Soc. 2009;131:9189. - PMC - PubMed
-
-
For selected examples that highlight this approach, see: Sharpless KB. Angew Chem Int Ed. 2002;41:2024.Johnson JS, Evans DA. Acc Chem Res. 2000;33:325.Fu GC. Acc Chem Res. 2000;33:412.Fulton JR, Holland AW, Fox DJ, Bergman RG. Acc Chem Res. 2002;35:44.Trost BM. Acc Chem Res. 2002;35:695.Saito S, Yamamoto H. Acc Chem Res. 2004;37:570.Enders D, Neimeier O, Henseler A. Chem Rev. 2007;107:5606.MacMillan DWC. Nature. 2008;455:304.Doyle AG, Jacobsen EN. Chem Rev. 2007;107:5713.
-
Grants and funding
LinkOut - more resources
Full Text Sources
