Homeostatic regulation of the endoneurial microenvironment during development, aging and in response to trauma, disease and toxic insult
- PMID: 21136068
- PMCID: PMC3038236
- DOI: 10.1007/s00401-010-0783-x
Homeostatic regulation of the endoneurial microenvironment during development, aging and in response to trauma, disease and toxic insult
Abstract
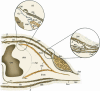
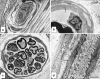
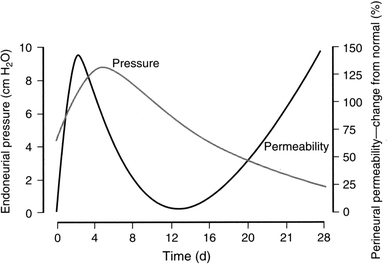
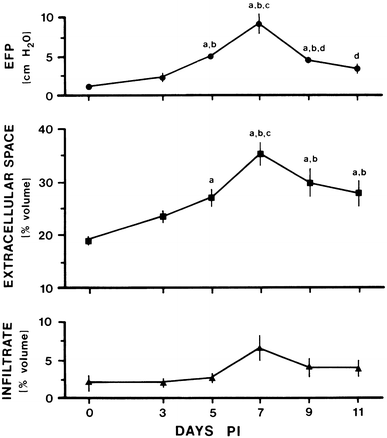
The endoneurial microenvironment, delimited by the endothelium of endoneurial vessels and a multi-layered ensheathing perineurium, is a specialized milieu intérieur within which axons, associated Schwann cells and other resident cells of peripheral nerves function. The endothelium and perineurium restricts as well as regulates exchange of material between the endoneurial microenvironment and the surrounding extracellular space and thus is more appropriately described as a blood-nerve interface (BNI) rather than a blood-nerve barrier (BNB). Input to and output from the endoneurial microenvironment occurs via blood-nerve exchange and convective endoneurial fluid flow driven by a proximo-distal hydrostatic pressure gradient. The independent regulation of the endothelial and perineurial components of the BNI during development, aging and in response to trauma is consistent with homeostatic regulation of the endoneurial microenvironment. Pathophysiological alterations of the endoneurium in experimental allergic neuritis (EAN), and diabetic and lead neuropathy are considered to be perturbations of endoneurial homeostasis. The interactions of Schwann cells, axons, macrophages, and mast cells via cell-cell and cell-matrix signaling regulate the permeability of this interface. A greater knowledge of the dynamic nature of tight junctions and the factors that induce and/or modulate these key elements of the BNI will increase our understanding of peripheral nerve disorders as well as stimulate the development of therapeutic strategies to treat these disorders.
Figures

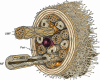

References
-
- Aguayo AJ, Charron L, Bray GM. Potential of Schwann cells from unmyelinated nerves to produce myelin: a quantitative ultrastructural and autoradiographic study. J Neurocytol. 1976;5:565–573. - PubMed
-
- Aijaz S, Balda MS, Matter K. Tight junctions: molecular architecture and function. Int Rev Cytol. 2006;248:261–298. - PubMed
-
- Allt G, Lawrenson JG. The blood–nerve barrier: enzymes, transporters and receptors—a comparison with the blood–brain barrier. Brain Res Bull. 2000;52:1–12. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

