Contribution of laser microdissection-based technology to proteomic analysis in hepatocellular carcinoma developing on cirrhosis
- PMID: 21136705
- PMCID: PMC3035364
- DOI: 10.1002/prca.200600474
Contribution of laser microdissection-based technology to proteomic analysis in hepatocellular carcinoma developing on cirrhosis
Abstract
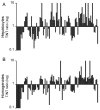
Hepatocellular carcinoma (HCC) is a major cause of cancer worldwide. Proteomic studies provide opportunities to uncover targets for the diagnosis and treatment of this disease. However, in HCC developing in a setting of cirrhosis, the detection of proteome alterations may be hampered by the increased cellular heterogeneity of tissue when analysing global liver homogenates. The aim of this study was to evaluate whether the identification of proteome alterations in these HCC cases was improved when the differential protein profile between tumour and non-tumour areas of liver was determined using hepatocytes isolated by laser microdissection (LM). Differential profiles established with LM-hepatocytes and liver section homogenates using 2-DE and MS exhibited noticeable differences: 30% of the protein spots with deregulated expression in tumorous LM-samples did not display any modification in homogenates; conversely 15% of proteins altered in tumorous homogenates were not impaired in LM-hepatocytes. These alterations resulted from the presence in cirrhotic liver of fibrotic stroma which displayed a protein pattern different from that determined in LM-hepatocytes. In conclusion, our data demonstrate the interest of LM in distinguishing between fibrotic and hepatocyte proteome alterations and thus the benefit of LM to proteome studies of HCC developing in a context of cirrhosis.
Copyright © 2007 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures




Similar articles
-
Laser capture microdissection in comparative proteomic analysis of hepatocellular carcinoma.Methods Cell Biol. 2007;82:689-707. doi: 10.1016/S0091-679X(06)82025-X. Methods Cell Biol. 2007. PMID: 17586277
-
Proteome analysis of hepatocellular carcinoma by laser capture microdissection.Proteomics. 2006 Jan;6(2):538-46. doi: 10.1002/pmic.200500257. Proteomics. 2006. PMID: 16342242
-
Laser microdissection for gene expression study of hepatocellular carcinomas arising in cirrhotic and non-cirrhotic livers.Methods Mol Biol. 2011;755:233-44. doi: 10.1007/978-1-61779-163-5_19. Methods Mol Biol. 2011. PMID: 21761308
-
Hepatocellular carcinoma: from bedside to proteomics.Proteomics. 2001 Oct;1(10):1249-63. doi: 10.1002/1615-9861(200110)1:10<1249::AID-PROT1249>3.0.CO;2-1. Proteomics. 2001. PMID: 11721636 Review.
-
[Epidemiology and diagnosis of hepatocellular carcinomas in cirrhosis].Ann Chir. 1998;52(6):511-7. Ann Chir. 1998. PMID: 9752500 Review. French.
Cited by
-
Enrichment of single neurons and defined brain regions from human brain tissue samples for subsequent proteome analysis.J Neural Transm (Vienna). 2015 Jul;122(7):993-1005. doi: 10.1007/s00702-015-1414-4. Epub 2015 Jun 30. J Neural Transm (Vienna). 2015. PMID: 26123835
-
Identification of cellular targets in human intrahepatic cholangiocarcinoma using laser microdissection and accurate mass and time tag proteomics.Mol Cell Proteomics. 2010 Sep;9(9):1991-2004. doi: 10.1074/mcp.M110.000026. Epub 2010 May 31. Mol Cell Proteomics. 2010. PMID: 20513801 Free PMC article.
-
KEGG spider: interpretation of genomics data in the context of the global gene metabolic network.Genome Biol. 2008;9(12):R179. doi: 10.1186/gb-2008-9-12-r179. Epub 2008 Dec 18. Genome Biol. 2008. PMID: 19094223 Free PMC article.
References
-
- Ferlay J, Bray F, Pisani P, Parkin D. IARC CancerBase N°5. 2004.
-
- Jemal A, Murray T, Ward E, Samuels A, et al. CA Cancer J Clin. 2005;55:10–30. - PubMed
-
- Johnson PJ. Clin Liver Dis. 2001;5:145–159. - PubMed
-
- Chignard N, Beretta L. Gastroenterology. 2004;127:S120–125. - PubMed
-
- Hanash S. Nature. 2003;422:226–232. - PubMed

