Prediction of the responsiveness to pharmacological chaperones: lysosomal human alpha-galactosidase, a case of study
- PMID: 21138548
- PMCID: PMC3016270
- DOI: 10.1186/1750-1172-5-36
Prediction of the responsiveness to pharmacological chaperones: lysosomal human alpha-galactosidase, a case of study
Abstract
Background: The pharmacological chaperones therapy is a promising approach to cure genetic diseases. It relies on substrate competitors used at sub-inhibitory concentration which can be administered orally, reach difficult tissues and have low cost. Clinical trials are currently carried out for Fabry disease, a lysosomal storage disorder caused by inherited genetic mutations of alpha-galactosidase. Regrettably, not all genotypes respond to these drugs.
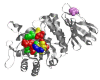
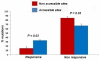
Results: We collected the experimental data available in literature on the enzymatic activity of ninety-six missense mutants of lysosomal alpha-galactosidase measured in the presence of pharmacological chaperones. We associated with each mutation seven features derived from the analysis of 3D-structure of the enzyme, two features associated with their thermo-dynamic stability and four features derived from sequence alone. Structural and thermodynamic analysis explains why some mutants of human lysosomal alpha-galactosidase cannot be rescued by pharmacological chaperones: approximately forty per cent of the non responsive cases examined can be correctly associated with a negative prognostic feature. They include mutations occurring in the active site pocket, mutations preventing disulphide bridge formation and severely destabilising mutations. Despite this finding, prediction of mutations responsive to pharmacological chaperones cannot be achieved with high accuracy relying on combinations of structure- and thermodynamic-derived features even with the aid of classical and state of the art statistical learning methods.We developed a procedure to predict responsive mutations with an accuracy as high as 87%: the method scores the mutations by using a suitable position-specific substitution matrix. Our approach is of general applicability since it does not require the knowledge of 3D-structure but relies only on the sequence.
Conclusions: Responsiveness to pharmacological chaperones depends on the structural/functional features of the disease-associated protein, whose complex interplay is best reflected on sequence conservation by evolutionary pressure. We propose a predictive method which can be applied to screen novel mutations of alpha galactosidase. The same approach can be extended on a genomic scale to find candidates for therapy with pharmacological chaperones among proteins with unknown tertiary structures.
Figures

References
-
- Flanagan JJ, Rossi B, Tang K, Wu X, Mascioli K, Donaudy F, Tuzzi MR, Fontana F, Cubellis MV, Porto C. et al. The pharmacological chaperone 1-deoxynojirimycin increases the activity and lysosomal trafficking of multiple mutant forms of acid alpha-glucosidase. Hum Mutat. 2009;30:1683–92. doi: 10.1002/humu.21121. - DOI - PubMed
-
- Caciotti A, Donati MA, d'Azzo A, Salvioli R, Guerrini R, Zammarchi E, Morrone A. The potential action of galactose as a "chemical chaperone": increase of beta galactosidase activity in fibroblasts from an adult GM1-gangliosidosis patient. Eur J Paediatr Neurol. 2009;13:160–4. doi: 10.1016/j.ejpn.2008.03.004. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

