Targeted in vivo O-GlcNAc sensors reveal discrete compartment-specific dynamics during signal transduction
- PMID: 21138847
- PMCID: PMC3057821
- DOI: 10.1074/jbc.M110.191627
Targeted in vivo O-GlcNAc sensors reveal discrete compartment-specific dynamics during signal transduction
Abstract
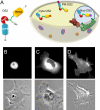
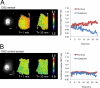
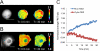
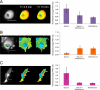
β-O-N-acetyl-D-glucosamine (O-GlcNAc) is a post-translational modification involved in a plethora of biological systems ranging from cellular stress to insulin signaling. This modification shares many hallmarks with phosphorylation, including its dynamic cycling onto a host of proteins such as transcription factors, kinases, and phosphatases, and regulation of cellular functions, including cell signaling. Herein, we report the development of an improved genetically based O-GlcNAc FRET sensor and compartmentalized targeted variants for the characterization of the spatiotemporal dynamics of O-GlcNAc. During serum-stimulated signal transduction, rapid increases in O-GlcNAc activity were observed at both the plasma membrane and the nucleus, with a concomitant decrease detected in the cytoplasm. These findings suggest the existence of compartment specific dynamics for O-GlcNAc in response to signal-inducing stimuli, pointing to complex regulation of this modification. In addition, inhibition of the PI3K pathway by wortmannin abolished the O-GlcNAc response, suggesting that the activity observed is modulated downstream of the PI3K pathway. Taken together, our data argues that O-GlcNAc is a rapidly induced component of signaling and that the interplay between O-GlcNAc and kinase signaling may be more akin to the complex relationship between kinase pathways.
Figures


References
-
- Yang X., Ongusaha P. P., Miles P. D., Havstad J. C., Zhang F., So W. V., Kudlow J. E., Michell R. H., Olefsky J. M., Field S. J., Evans R. M. (2008) Nature 451, 964–969 - PubMed
-
- Nanashima N., Asano J., Hayakari M., Nakamura T., Nakano H., Yamada T., Shimizu T., Akita M., Fan Y., Tsuchida S. (2005) J. Biol. Chem. 280, 43010–43016 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

