Development of human anti-murine T-cell receptor antibodies in both responding and nonresponding patients enrolled in TCR gene therapy trials
- PMID: 21138872
- PMCID: PMC3058233
- DOI: 10.1158/1078-0432.CCR-10-1280
Development of human anti-murine T-cell receptor antibodies in both responding and nonresponding patients enrolled in TCR gene therapy trials
Abstract
Purpose: Immune responses to gene-modified cells are a concern in the field of human gene therapy, as they may impede effective treatment. We conducted 2 clinical trials in which cancer patients were treated with lymphocytes genetically engineered to express murine T-cell receptors (mTCR) specific for tumor-associated antigens p53 and gp100.
Experimental design: Twenty-six patients treated with autologous lymphocytes expressing mTCR had blood and serum samples available for analysis. Patient sera were assayed for the development of a humoral immune response. Adoptive cell transfer characteristics were analyzed to identify correlates to immune response.
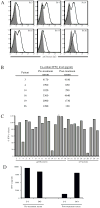
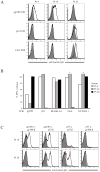
Results: Six of 26 (23%) patients' posttreatment sera exhibited specific binding of human anti-mTCR antibodies to lymphocytes transduced with the mTCR. Antibody development was found in both responding and nonresponding patients. The posttreatment sera of 3 of these 6 patients mediated a 60% to 99% inhibition of mTCR activity as measured by a reduction in antigen-specific interferon-γ release. Detailed analysis of posttreatment serum revealed that antibody binding was β-chain specific in 1 patient whereas it was α-chain specific in another.
Conclusions: A subset of patients treated with mTCR-engineered T cells developed antibodies directed to the mTCR variable regions and not to the constant region domains common to all mTCR. Overall, the development of a host immune response was not associated with the level of transduced cell persistence or response to therapy. In summary, patients treated with mTCR can develop an immune response to gene-modified cells in a minority of cases, but this may not affect clinical outcome.
©2010 AACR.
Conflict of interest statement
Figures
References
-
- Rosenberg SA, Aebersold P, Cornetta K, et al. Gene transfer into humans--immunotherapy of patients with advanced melanoma, using tumor-infiltrating lymphocytes modified by retroviral gene transduction. N Engl J Med. 1990;323:570–8. - PubMed
-
- Health NIo. Human Gene Transfer Protocols. 2010. [cited 2010 January 15]; Available from: http://oba.od.nih.gov/oba/rac/PROTOCOL.pdf.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

