Esrrg functions in early branch generation of the ureteric bud and is essential for normal development of the renal papilla
- PMID: 21138943
- PMCID: PMC3033182
- DOI: 10.1093/hmg/ddq530
Esrrg functions in early branch generation of the ureteric bud and is essential for normal development of the renal papilla
Abstract
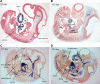
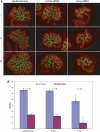
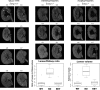
Congenital anomalies of the kidney and urinary tract (CAKUTs) are common disorders of human development affecting the renal parechyma, renal pelvis, ureter, bladder and urethra; they show evidence of shared genetic aetiology, although the molecular basis of this remains unknown in the majority of cases. Breakpoint mapping of a de novo, apparently balanced, reciprocal translocation associated with bilateral renal agenesis has implicated the gene encoding the nuclear steroid hormone receptor ESRRG as a candidate gene for CAKUT. Here we show that the Esrrg protein is detected throughout early ureteric ducts as cytoplasmic/sub-membranous staining; with nuclear localization seen in developing nephrons. In 14.5-16.5 dpc (days post-conception) mouse embryos, Esrrg localizes to the subset of ductal tissue within the kidney, liver and lung. The renal ductal expression becomes localized to renal papilla by 18.5 dpc. Perturbation of function was performed in embryonic mouse kidney culture using pooled siRNA to induce knock-down and a specific small-molecule agonist to induce aberrant activation of Esrrg. Both resulted in severe abnormality of early branching events of the ureteric duct. Mouse embryos with a targeted inactivation of Esrrg on both alleles (Esrrg(-/-)) showed agenesis of the renal papilla but normal development of the cortex and remaining medulla. Taken together, these results suggest that Esrrg is required for early branching events of the ureteric duct that occur prior to the onset of nephrogenesis. These findings confirm ESRRG as a strong candidate gene for CAKUT.
Figures





References
-
- Hiruma T., Nakamura H. Origin and development of the pronephros in the chick embryo. J. Anat. 2003;203:539–552. doi:10.1046/j.1469-7580.2003.00245.x. - DOI - PMC - PubMed
-
- O'Rahilly R., Müller F. Developmental Stages in Human Embryos. Carnegie Institution of Washington; 1987.
-
- Costantini F. Renal branching morphogenesis: concepts, questions, and recent advances. Differentiation. 2006;74:402–421. doi:10.1111/j.1432-0436.2006.00106.x. - DOI - PubMed
-
- Dressler G.R. The cellular basis of kidney development. Annu. Rev. Cell Dev. Biol. 2006;22:509–529. doi:10.1146/annurev.cellbio.22.010305.104340. - DOI - PubMed
-
- Woolf A.S., Price K.L., Scambler P.J., Winyard P.J. Evolving concepts in human renal dysplasia. J. Am. Soc. Nephrol. 2004;15:998–1007. doi:10.1097/01.ASN.0000113778.06598.6F. - DOI - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

