Retinoic acid stimulates myocardial expansion by induction of hepatic erythropoietin which activates epicardial Igf2
- PMID: 21138976
- PMCID: PMC2998168
- DOI: 10.1242/dev.054239
Retinoic acid stimulates myocardial expansion by induction of hepatic erythropoietin which activates epicardial Igf2
Abstract
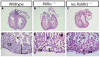
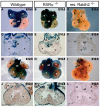
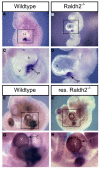
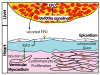
Epicardial signaling and Rxra are required for expansion of the ventricular myocardial compact zone. Here, we examine Raldh2(-/-) and Rxra(-/-) mouse embryos to investigate the role of retinoic acid (RA) signaling in this developmental process. The heart phenotypes of Raldh2 and Rxra mutants are very similar and are characterized by a prominent defect in ventricular compact zone growth. Although RA activity is completely lost in Raldh2(-/-) epicardium and the adjacent myocardium, RA activity is not lost in Rxra(-/-) hearts, suggesting that RA signaling in the epicardium/myocardium is not required for myocardial compact zone formation. We explored the possibility that RA-mediated target gene transcription in non-cardiac tissues is required for this process. We found that hepatic expression of erythropoietin (EPO), a secreted factor implicated in myocardial expansion, is dependent on both Raldh2 and Rxra. Chromatin immunoprecipitation studies support Epo as a direct target of RA signaling in embryonic liver. Treatment of an epicardial cell line with EPO, but not RA, upregulates Igf2. Furthermore, both Raldh2(-/-) and Rxra(-/-) hearts exhibit downregulation of Igf2 mRNA in the epicardium. EPO treatment of cultured Raldh2(-/-) hearts restores epicardial Igf2 expression and rescues ventricular cardiomyocyte proliferation. We propose a new model for the mechanism of RA-mediated myocardial expansion in which RA directly induces hepatic Epo resulting in activation of epicardial Igf2 that stimulates compact zone growth. This RA-EPO-IGF2 signaling axis coordinates liver hematopoiesis with heart development.
Figures





References
-
- Azambuja A. P., Portillo-Sanchez V., Rodrigues M. V., Omae S. V., Schechtman D., Strauss B. E., Costanzi-Strauss E., Krieger J. E., Perez-Pomares J. M., Xavier-Neto J. (2010). Retinoic Acid and VEGF delay smooth muscle relative to endothelial differentiation to coordinate inner and outer coronary vessel wall morphogenesis. Circ. Res. 107, 204-216 - PubMed
-
- Chen J., Kubalak S. W., Chien K. R. (1998). Ventricular muscle-restricted targeting of the RXRα gene reveals a non-cell-autonomous requirement in cardiac chamber morphogenesis. Development 125, 1943-1949 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

