Soluble FLT1 sensitizes endothelial cells to inflammatory cytokines by antagonizing VEGF receptor-mediated signalling
- PMID: 21139021
- PMCID: PMC3028975
- DOI: 10.1093/cvr/cvq346
Soluble FLT1 sensitizes endothelial cells to inflammatory cytokines by antagonizing VEGF receptor-mediated signalling
Abstract
Aims: Pre-eclampsia affects 5-7% of pregnancies, and is a major cause of maternal and foetal death. Elevated serum levels of placentally derived splice variants of the vascular endothelial growth factor (VEGF) receptor, soluble fms-like tyrosine kinase-1 (sFLT1), are strongly implicated in the pathogenesis but, as yet, no underlying mechanism has been described. An excessive inflammatory-like response is thought to contribute to the maternal endothelial cell dysfunction that characterizes pre-eclampsia. We hypothesized that sFLT1 antagonizes autocrine VEGF-A signalling, rendering endothelial cells more sensitive to pro-inflammatory factors also released by the placenta. We tested this by manipulating VEGF receptor signalling and treating endothelial cells with low doses of tumour necrosis factor-α (TNF-α).
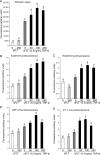
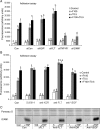
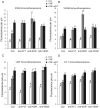
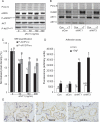
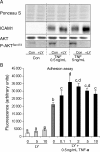
Methods and results: Application of recombinant sFLT1 alone did not activate human umbilical vein endothelial cells (HUVECs). However, antagonizing the autocrine actions of endothelial VEGF-A and/or placenta growth factor (PlGF) by pre-incubation with recombinant sFLT1, anti-FLT1, anti-VEGF receptor 2 (KDR), anti-VEGF-A, VEGF receptor tyrosine kinase inhibitor SU5614, or knocking-down FLT1 or KDR transcripts rendered cells more sensitive to low doses of TNF-α. Each treatment increased activation, as measured by increases in endothelial intercellular adhesion molecule 1 (ICAM1), vascular cell adhesion molecule 1 (VCAM1), endothelin 1 (ET-1), von Willebrand factor (vWF), and leucocyte adhesion, and led to reduction in AKT Ser⁴⁷³ and endothelial nitric oxide synthase (eNOS) Ser¹¹⁷⁷ phosphorylation.
Conclusions: Our data describe a mechanism by which sFLT1 sensitizes endothelial cells to pro-inflammatory factors, providing an explanation for how placental stress may precipitate the pre-eclamptic syndrome.
Figures
References
-
- Bethesda M WHO. Make Every Mother and Child Count. National Institutes of Health: WHO; 2005. Global burden of disease for the year 2001 by World Bank region, for use in disease control priorities in developing countries. WHRGWHOW, 2004. http://www.who.int/whr/2005/chapter3/en/index2.html .
-
- Redman CW, Sargent IL. Latest advances in understanding preeclampsia. Science. 2005;308:1592–1594. doi:10.1126/science.1111726. - DOI - PubMed
-
- Roberts JM. Endothelial dysfunction in preeclampsia. Semin Reprod Endocrinol. 1998;16:5–15. doi:10.1055/s-2007-1016248. - DOI - PubMed
-
- Myatt L, Webster RP. Vascular biology of preeclampsia. J Thromb Haemost. 2009;7:375–384. doi:10.1111/j.1538-7836.2008.03259.x. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

