A kinase-independent role for unoccupied insulin and IGF-1 receptors in the control of apoptosis
- PMID: 21139139
- PMCID: PMC4364396
- DOI: 10.1126/scisignal.2001173
A kinase-independent role for unoccupied insulin and IGF-1 receptors in the control of apoptosis
Abstract
Insulin and insulin-like growth factor 1 (IGF-1) act as antiapoptotic hormones. We found that, unexpectedly, double-knockout (DKO) cells that lacked both insulin and IGF-1 receptors (IR and IGF1R, respectively) were resistant to apoptosis induced through either the intrinsic or the extrinsic pathway. This resistance to apoptosis was associated with decreased abundance of the proapoptotic protein Bax and increases in abundance of the antiapoptotic proteins Bcl-2, Bcl-xL, XIAP, and Flip. These changes in protein abundance involved primarily posttranscriptional mechanisms. Restoration of IR or IGF1R to DKO cells also restored their sensitivity to apoptosis. Notably, expression of a catalytically inactive mutant form of the IR also restored susceptibility to apoptosis. Thus, IR and IGF1R have bidirectional roles in the control of cell survival and can be viewed as dependence receptors. Insulin and IGF-1 binding stimulates receptor tyrosine kinase activity and blocks apoptosis, whereas unliganded IR and IGF1R, acting through a mechanism independent of their catalytic activity, exert a permissive effect on cell death.
Figures
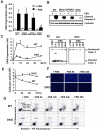


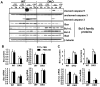



References
-
- Arends MJ, Wyllie AH. Apoptosis: mechanisms and roles in pathology. Int.Rev.Exp Pathol. 1991;32:223–254. - PubMed
-
- Thompson CB. Apoptosis in the pathogenesis and treatment of disease. Science. 1995;267:1456–1462. - PubMed
-
- Nicholson DW. ICE/CED3-like proteases as therapeutic targets for the control of inappropriate apoptosis. Nat.Biotechnol. 1996;14:297–301. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

