Progress in the structural understanding of voltage-gated calcium channel (CaV) function and modulation
- PMID: 21139419
- PMCID: PMC3018750
- DOI: 10.4161/chan.4.6.12867
Progress in the structural understanding of voltage-gated calcium channel (CaV) function and modulation
Abstract
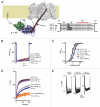
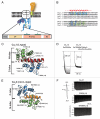
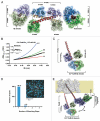
Voltage-gated calcium channels (CaVs) are large, transmembrane multiprotein complexes that couple membrane depolarization to cellular calcium entry. These channels are central to cardiac action potential propagation, neurotransmitter and hormone release, muscle contraction, and calcium-dependent gene transcription. Over the past six years, the advent of high-resolution structural studies of CaV components from different isoforms and CaV modulators has begun to reveal the architecture that underlies the exceptionally rich feedback modulation that controls CaV action. These descriptions of CaV molecular anatomy have provided new, structure-based insights into the mechanisms by which particular channel elements affect voltage-dependent inactivation (VDI), calcium‑dependent inactivation (CDI), and calcium‑dependent facilitation (CDF). The initial successes have been achieved through structural studies of soluble channel domains and modulator proteins and have proven most powerful when paired with biochemical and functional studies that validate ideas inspired by the structures. Here, we review the progress in this growing area and highlight some key open challenges for future efforts.
Figures
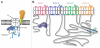



References
-
- Hille B. Ion Channels of Excitable Membranes. Sunderland, MA: Sinauer Associates, Inc; 2001.
-
- Catterall WA. Structure and regulation of voltage-gated Ca2+ channels. Annu Rev Cell Dev Biol. 2000;16:521–555. - PubMed
-
- Catterall WA, Perez-Reyes E, Snutch TP, Striessnig J. International Union of Pharmacology. XLVIII. Nomenclature and structure-function relationships of voltage-gated calcium channels. Pharmacol Rev. 2005;57:411–425. - PubMed
-
- Clapham DE. Calcium signaling. Cell. 2007;131:1047–1058. - PubMed
-
- Ashcroft FM. From molecule to malady. Nature. 2006;440:440–447. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
