In vivo 3D morphology of astrocyte-vasculature interactions in the somatosensory cortex: implications for neurovascular coupling
- PMID: 21139630
- PMCID: PMC3063633
- DOI: 10.1038/jcbfm.2010.204
In vivo 3D morphology of astrocyte-vasculature interactions in the somatosensory cortex: implications for neurovascular coupling
Abstract
Astrocytes are increasingly believed to play an important role in neurovascular coupling. Recent in vivo studies have shown that intracellular calcium levels in astrocytes correlate with reactivity in adjacent diving arterioles. However, the hemodynamic response to stimulation involves a complex orchestration of vessel dilations and constrictions that spread rapidly over wide distances. In this work, we study the three-dimensional cytoarchitecture of astrocytes and their interrelations with blood vessels down through layer IV of the mouse somatosensory cortex using in vivo two-photon microscopy. Vessels and astrocytes were visualized through intravenous dextran-conjugated fluorescein and cortically applied sulforhodamine 101 (SR101), respectively. In addition to exploring astrocyte density, vascular proximity, and microvascular density, we found that sheathing of subpial vessels by astrocyte processes was continuous along all capillaries, arterioles, and veins, comprising a highly interconnected pathway through which signals could feasibly be relayed over long distances via gap junctions. An inner SR101-positive sheath noted along pial and diving arterioles was determined to be nonastrocytic, and appears to represent selective SR101 staining of arterial endothelial cells. Our findings underscore the intimate relationship between astrocytes and all cortical blood vessels, and suggest that astrocytes could influence neurovascular regulation at a range of sites, including the capillary bed and pial arterioles.
Figures
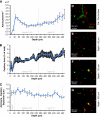


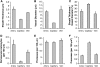

Comment in
-
Glial laminar cortical architecture matches metabolic demand.J Cereb Blood Flow Metab. 2011 Mar;31(3):793-4. doi: 10.1038/jcbfm.2010.205. Epub 2010 Dec 8. J Cereb Blood Flow Metab. 2011. PMID: 21139627 Free PMC article. No abstract available.
References
-
- Altamura C, Dell'Acqua MI, Moessner R, Murphy DI, Lesch KP, Persico AM. Altered neocortical cell density and layer thickness in serotonin transporter knockout mice: a quantitation study. Cerebral Cortex. 2007;17:1394–1401. - PubMed
-
- Andresen J, Shafi NI, Bryan RM. Endothelial influences on cerebrovascular tone. J Appl Physiol. 2006;100:318–327. - PubMed
-
- Borowsky IW, Collins RC. Metabolic anatomy of brain: a comparison of regional capillary density, glucose metabolism, and enzyme activities. J Comp Neurol. 1989;288:401–413. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

