Regeneration of corneal endothelium following complete endothelial cell loss in rat keratoplasty
- PMID: 21139971
- PMCID: PMC2994736
Regeneration of corneal endothelium following complete endothelial cell loss in rat keratoplasty
Abstract
Purpose: Corneal endothelial cells (EC) are crucial for maintaining corneal clarity before and after keratoplasty. Since it is thought that corneal graft rejection leads to irreversible EC loss and transplant failure, we quantified immune mediated EC loss in the rat keratoplasty model and analyzed whether the EC layer would then regenerate.
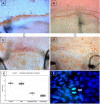
Methods: Rats were subjected to orthotopic penetrating keratoplasty. We compared endothelial responses to immunological EC loss following allogeneic transplantations between Fisher and Lewis rats (group R) to those following mechanical EC removal in a syngeneic setting between Lewis rats (group S). Animals were followed clinically for corneal opacity for up to one year. Bulbi were excised and prepared for histological examination at different time points: ECs were defined and characterized using Alicarin red S/ DAPI staining on corneal flatmounts. Ki-67/ DAPI staining on flatmount preparations served to detect cell proliferation. Immunohistochemical staining of corneal cryosections was used to characterize infiltrating immune cells.
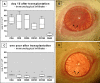
Results: GROUP R: After about two weeks the allografts were completely opaque, which was accompanied by a massive leukocyte infiltration in conjunction with EC destruction, signifying rejection. EC loss without an immune reaction (group S) resulted only in medium opacity levels. In both groups, all grafts regained clarity in the following weeks to months, and a newly-formed endothelial cell layer with irregular and enlarged ECs became apparent on the formerly EC free grafts. Scattered Ki-67 positive cells within the endothelial cell layer were observed during re-endothelialization. In addition to re-endothelialization, the immunological infiltration seen in the allografts at the time of rejection had subsided after one year.
Conclusions: Re-endothelialization following keratoplasty takes place in the rat in vivo and restores graft clarity, following both immunological or surgical destruction of ECs. Following rejection, EC replacement is accompanied by a reduction of immune infiltrates. Peripheral recipient ECs are a sufficient source for graft re-endothelialization, as seen in rats following EC removal. Our results suggest that ECs both proliferate and enlarge during re-endothelialization in the rat keratoplasty model.
Figures

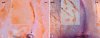

Similar articles
-
Regeneration of corneal endothelial cells following keratoplasty in rats with bullous keratopathy.Mol Vis. 2014 May 27;20:683-90. eCollection 2014. Mol Vis. 2014. PMID: 24883013 Free PMC article.
-
Host-derived endothelial regeneration of corneal transplants in a rat keratoplasty model.Ophthalmic Res. 2014;52(2):60-4. doi: 10.1159/000360739. Epub 2014 Jul 1. Ophthalmic Res. 2014. PMID: 24993185
-
Corneal allograft endothelial cell replacement represents a reparative response to transplant injury.Mol Vis. 2009;15:654-61. Epub 2009 Apr 3. Mol Vis. 2009. PMID: 19347050 Free PMC article.
-
[Corneal transplantation].Zentralbl Chir. 1992;117(12):695-700. Zentralbl Chir. 1992. PMID: 1285478 Review. German.
-
[Rejection of corneal allografts].J Fr Ophtalmol. 2011 May;34(5):331-48. doi: 10.1016/j.jfo.2011.02.001. Epub 2011 Apr 14. J Fr Ophtalmol. 2011. PMID: 21492957 Review. French.
Cited by
-
Corneal endothelial regeneration in human eyes using endothelium-free grafts.BMC Ophthalmol. 2022 Jan 21;22(1):32. doi: 10.1186/s12886-022-02260-x. BMC Ophthalmol. 2022. PMID: 35062892 Free PMC article.
-
Regeneration of corneal endothelial cells following keratoplasty in rats with bullous keratopathy.Mol Vis. 2014 May 27;20:683-90. eCollection 2014. Mol Vis. 2014. PMID: 24883013 Free PMC article.
-
Corneal endothelial transplantation from bench to bedside: A review of animal models and their translational value for therapeutic development.Exp Eye Res. 2022 Nov;224:109241. doi: 10.1016/j.exer.2022.109241. Epub 2022 Sep 6. Exp Eye Res. 2022. PMID: 36075460 Free PMC article. Review. No abstract available.
-
Progenitors for the corneal endothelium and trabecular meshwork: a potential source for personalized stem cell therapy in corneal endothelial diseases and glaucoma.J Biomed Biotechnol. 2011;2011:412743. doi: 10.1155/2011/412743. Epub 2011 Dec 6. J Biomed Biotechnol. 2011. PMID: 22187525 Free PMC article. Review.
-
From DMEK to Corneal Endothelial Cell Therapy: Technical and Biological Aspects.J Ophthalmol. 2018 Aug 1;2018:6482095. doi: 10.1155/2018/6482095. eCollection 2018. J Ophthalmol. 2018. PMID: 30155283 Free PMC article. Review.
References
-
- Reinhard T, Böhringer D, Enczmann J, Kogler G, Wernet P, Böhringer S, Sundmacher R. HLA class I/II matching and chronic endothelial cell loss in penetrating normal risk keratoplasty. Acta Ophthalmol Scand. 2004;82:13–8. - PubMed
-
- Bourne WM, Hodge DO, Nelson LR. Corneal endothelium five years after transplantation. Am J Ophthalmol. 1994;118:185–96. - PubMed
-
- Ing JJ, Ing HH, Nelson LR, Hodge DO, Bourne WM. Ten-year postoperative results of penetrating keratoplasty. Ophthalmology. 1998;105:1855–65. - PubMed
-
- Rose L, Kelliher C, Jun AS. Endothelial keratoplasty: historical perspectives, current techniques, future directions. Can J Ophthalmol. 2009;44:401–5. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
