HSV-1 infection of human corneal epithelial cells: receptor-mediated entry and trends of re-infection
- PMID: 21139972
- PMCID: PMC2994737
HSV-1 infection of human corneal epithelial cells: receptor-mediated entry and trends of re-infection
Abstract
Purpose: The human cornea is a primary target for herpes simplex virus-1 (HSV-1) infection. The goals of the study were to determine the cellular modalities of HSV-1 entry into human corneal epithelial (HCE) cells. Specific features of the study included identifying major entry receptors, assessing pH dependency, and determining trends of re-infection.
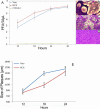
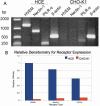
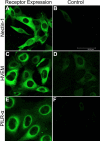
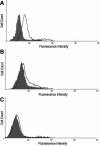
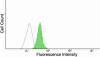
Methods: A recombinant HSV-1 virus expressing beta-galactosidase was used to ascertain HSV-1 entry into HCE cells. Viral replication within cells was confirmed using a time point plaque assay. Lysosomotropic agents were used to test for pH dependency of entry. Flow cytometry and immunocytochemistry were used to determine expression of three cellular receptors--nectin-1, herpesvirus entry mediator (HVEM), and paired immunoglobulin-like 2 receptor alpha (PILR-a). The necessity of these receptors for viral entry was tested using antibody-blocking. Finally, trends of re-infection were investigated using viral entry assay and flow cytometry post-primary infection.
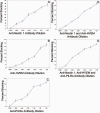
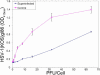
Results: Cultured HCE cells showed high susceptibility to HSV-1 entry and replication. Entry was demonstrated to be pH dependent as blocking vesicular acidification decreased entry. Entry receptors expressed on the cell membrane include nectin-1, HVEM, and PILR-α. Receptor-specific antibodies blocked entry receptors, reduced viral entry and indicated nectin-1 as the primary receptor used for entry. Cells re-infected with HSV-1 showed a decrease in entry, which was correlated to decreased levels of nectin-1 as demonstrated by flow cytometry.
Conclusions: HSV-1 is capable of developing an infection in HCE cells using a pH dependent entry process that involves primarily nectin-1 but also the HVEM and PILR-α receptors. Re-infected cells show decreased levels of entry, correlated with a decreased level of nectin-1 receptor expression.
Figures


Similar articles
-
Herpesvirus Entry into Host Cells Mediated by Endosomal Low pH.Traffic. 2016 Sep;17(9):965-75. doi: 10.1111/tra.12408. Epub 2016 May 24. Traffic. 2016. PMID: 27126894 Free PMC article. Review.
-
Role of nectin-1, HVEM, and PILR-alpha in HSV-2 entry into human retinal pigment epithelial cells.Invest Ophthalmol Vis Sci. 2009 Jun;50(6):2878-87. doi: 10.1167/iovs.08-2981. Epub 2009 Feb 21. Invest Ophthalmol Vis Sci. 2009. PMID: 19234349 Free PMC article.
-
Nectin-1-specific entry of herpes simplex virus 1 is sufficient for infection of the cornea and viral spread to the trigeminal ganglia.Mol Vis. 2012;18:2711-6. Epub 2012 Nov 16. Mol Vis. 2012. PMID: 23213272 Free PMC article.
-
HVEM and nectin-1 are the major mediators of herpes simplex virus 1 (HSV-1) entry into human conjunctival epithelium.Invest Ophthalmol Vis Sci. 2008 Sep;49(9):4026-35. doi: 10.1167/iovs.08-1807. Epub 2008 May 23. Invest Ophthalmol Vis Sci. 2008. PMID: 18502984 Free PMC article.
-
Herpesvirus Entry Mediator and Ocular Herpesvirus Infection: More than Meets the Eye.J Virol. 2017 Jun 9;91(13):e00115-17. doi: 10.1128/JVI.00115-17. Print 2017 Jul 1. J Virol. 2017. PMID: 28404853 Free PMC article. Review.
Cited by
-
Heparanase is a host enzyme required for herpes simplex virus-1 release from cells.Nat Commun. 2015 Apr 27;6:6985. doi: 10.1038/ncomms7985. Nat Commun. 2015. PMID: 25912399 Free PMC article.
-
Herpes keratitis.Prog Retin Eye Res. 2013 Jan;32:88-101. doi: 10.1016/j.preteyeres.2012.08.002. Epub 2012 Aug 27. Prog Retin Eye Res. 2013. PMID: 22944008 Free PMC article. Review.
-
Targeting Herpes Simplex Virus-1 gD by a DNA Aptamer Can Be an Effective New Strategy to Curb Viral Infection.Mol Ther Nucleic Acids. 2017 Dec 15;9:365-378. doi: 10.1016/j.omtn.2017.10.009. Epub 2017 Oct 17. Mol Ther Nucleic Acids. 2017. PMID: 29246315 Free PMC article.
-
HSV-1 inhibits melanogenesis of PIG1 cells through downregulation of VN1R5/ERK pathway.Arch Dermatol Res. 2025 Feb 6;317(1):347. doi: 10.1007/s00403-025-03844-5. Arch Dermatol Res. 2025. PMID: 39912936
-
Herpesvirus Entry into Host Cells Mediated by Endosomal Low pH.Traffic. 2016 Sep;17(9):965-75. doi: 10.1111/tra.12408. Epub 2016 May 24. Traffic. 2016. PMID: 27126894 Free PMC article. Review.
References
-
- Toma HS, Murina AT, Areaux RG, Jr, Neumann DM, Bhattacharjee PS, Foster TP, Kaufman HE, Hill J. Ocular HSV-1 latency, reactivation and recurrent disease. Semin Ophthalmol. 2008;23:249–73. - PubMed
-
- Liesegang TJ. Herpes simplex virus epidemiology and ocular importance. Cornea. 2001;20:1–13. - PubMed
-
- Knickelbein JE, Hendricks RL, Charukamnoetkanok P. Management of herpes simplex virus stromal keratitis: an evidence-based review. Surv Ophthalmol. 2009;54:226–34. - PubMed
-
- Kaye S, Choudhary A. Herpes simplex keratitis. Prog Retin Eye Res. 2006;25:355–80. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
