Gap junctions are selectively associated with interlocking ball-and-sockets but not protrusions in the lens
- PMID: 21139982
- PMCID: PMC2994765
Gap junctions are selectively associated with interlocking ball-and-sockets but not protrusions in the lens
Abstract
Purpose: Ball-and-sockets and protrusions are specialized interlocking membrane domains between lens fibers of all species studied. Ball-and-sockets and protrusions are similar in their shape, size, and surface morphology, and are traditionally believed to play a key role in maintaining fiber-to-fiber stability. Here, we evaluate the hypothesis that ball-and-sockets and protrusions possess important structural and functional differences during fiber cell differentiation and maturation.
Methods: Intact lenses of leghorn chickens (E7 days to P62 weeks old) and rhesus monkeys (1.5-20 years old) were studied with SEM, freeze-fracture TEM, freeze-fracture immunogold labeling (FRIL), and filipin cytochemistry for membrane cholesterol detection.
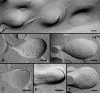
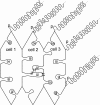
Results: SEM showed that ball-and-sockets were distributed along the long and short sides of hexagonal fiber cells, whereas protrusions were located along the cell corners, from superficial to deep cortical regions in both chicken and monkey lenses. Importantly, by freeze-fracture TEM, we discovered the selective association of gap junctions with all ball-and-sockets examined, but not with protrusions, in both species. In the embryonic chicken lens (E18), the abundant distribution of ball-and-socket gap junctions was regularly found in an approximate zone extending at least 300 μm deep from the equatorial surface of the superficial cortical fibers. Many ball-and-socket gap junctions often protruded deeply into neighboring cells. However, in the mature fibers of monkey lenses, several ball-and-sockets exhibited only partial occupancy of gap junctions with disorganized connexons, possibly due to degradation of gap junctions during fiber maturation and aging. FRIL analysis confirmed that both connexin46 (Cx46) and connexin50 (Cx50) antibodies specifically labeled ball-and-socket gap junctions, but not protrusions. Furthermore, filipin cytochemistry revealed that the ball-and-socket gap junctions contained different amounts of cholesterol (i.e., cholesterol-rich versus cholesterol-free) as seen with the filipin-cholesterol-complexes (FCC) in different cortical regions during maturation. In contrast, the protrusions contained consistently high cholesterol amounts (i.e., 402 FCCs/μm2 membrane) which were approximately two times greater than that of the cholesterol-rich gap junctions (i.e., 188 FCCs/μm2 membrane) found in ball-and-sockets.
Conclusions: Gap junctions are regularly associated with all ball-and-sockets examined in metabolically active young cortical fibers, but not with protrusions, in both chicken and monkey lenses. Since these unique gap junctions often protrude deeply into neighboring cells to increase membrane surface areas, they may significantly facilitate cell-to-cell communication between young cortical fiber cells. In particular, the large number of ball-and-socket gap junctions found near the equatorial region may effectively facilitate the flow of outward current toward the equatorial surface for internal circulation of ions in the lens. In contrast, a consistent distribution of high concentrations of cholesterol in protrusions would make the protrusion membrane less deformable and would be more suitable for maintaining fiber-to-fiber stability during visual accommodation. Thus, the ball-and-sockets and protrusions are two structurally and functionally distinct membrane domains in the lens.
Figures







Similar articles
-
Breakdown of interlocking domains may contribute to formation of membranous globules and lens opacity in ephrin-A5(-/-) mice.Exp Eye Res. 2016 Apr;145:130-139. doi: 10.1016/j.exer.2015.11.017. Epub 2015 Nov 28. Exp Eye Res. 2016. PMID: 26643403 Free PMC article.
-
Gap junction remodeling associated with cholesterol redistribution during fiber cell maturation in the adult chicken lens.Mol Vis. 2009 Aug 4;15:1492-508. Mol Vis. 2009. PMID: 19657477 Free PMC article.
-
Gap junctions contain different amounts of cholesterol which undergo unique sequestering processes during fiber cell differentiation in the embryonic chicken lens.Mol Vis. 2007 Mar 9;13:345-59. Mol Vis. 2007. PMID: 17392685 Free PMC article.
-
Primary cultures of embryonic chick lens cells as a model system to study lens gap junctions and fiber cell differentiation.J Membr Biol. 2012 Jul;245(7):357-68. doi: 10.1007/s00232-012-9458-y. Epub 2012 Jul 15. J Membr Biol. 2012. PMID: 22797938 Review.
-
[Aging changes in ocular tissues and their influences on accommodative functions].Nippon Ganka Gakkai Zasshi. 1990 Feb;94(2):93-119. Nippon Ganka Gakkai Zasshi. 1990. PMID: 2114735 Review. Japanese.
Cited by
-
Tissue, cellular, and molecular level determinants for eye lens stiffness and elasticity.Front Ophthalmol (Lausanne). 2024 Aug 8;4:1456474. doi: 10.3389/fopht.2024.1456474. eCollection 2024. Front Ophthalmol (Lausanne). 2024. PMID: 39176256 Free PMC article. Review.
-
Changes in the Properties and Organization of Human Lens Lipid Membranes Occurring with Age.Curr Eye Res. 2017 May;42(5):721-731. doi: 10.1080/02713683.2016.1231325. Epub 2016 Oct 28. Curr Eye Res. 2017. PMID: 27791387 Free PMC article.
-
Lipid domains in intact fiber-cell plasma membranes isolated from cortical and nuclear regions of human eye lenses of donors from different age groups.Exp Eye Res. 2015 Mar;132:78-90. doi: 10.1016/j.exer.2015.01.018. Epub 2015 Jan 21. Exp Eye Res. 2015. PMID: 25617680 Free PMC article.
-
Knock-in of Cx46 partially rescues fiber defects in lenses lacking Cx50.Mol Vis. 2017 Mar 24;23:160-170. eCollection 2017. Mol Vis. 2017. PMID: 28458505 Free PMC article.
-
Breakdown of interlocking domains may contribute to formation of membranous globules and lens opacity in ephrin-A5(-/-) mice.Exp Eye Res. 2016 Apr;145:130-139. doi: 10.1016/j.exer.2015.11.017. Epub 2015 Nov 28. Exp Eye Res. 2016. PMID: 26643403 Free PMC article.
References
-
- Rae JL. The electrophysiology of the crystalline lens. Curr Top Eye Res. 1979;1:37–90. - PubMed
-
- Goodenough DA. Lens gap junctions: a structural hypothesis for nonregulated low-resistance intercellular pathways. Invest Ophthalmol Vis Sci. 1979;18:1104–22. - PubMed
-
- Leeson TS. Lens of the rat eye: an electron microscope and freeze-etch study. Exp Eye Res. 1971;11:78–82. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
