Digitoxin activates EGR1 and synergizes with paclitaxel on human breast cancer cells
- PMID: 21139994
- PMCID: PMC2997271
- DOI: 10.4103/1477-3163.72578
Digitoxin activates EGR1 and synergizes with paclitaxel on human breast cancer cells
Abstract
Background: Numerous studies have suggested that digitalis derivatives promise to be superior to existing adjuvant therapy for breast cancer as to effects and side-effects. In the present study, we have used gene expression analysis to determine the molecular action of digitoxin on breast cancer cells and assessed digitoxin's ability to synergize with the chemotherapy agent paclitaxel with respect to inhibition of cell proliferation
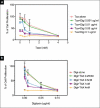
Materials and methods: We treated (Her2 overexpressing, ER low) MDA-MB-453 human breast cancer cells with digitoxin at four doses {20 ng/ml (26 nM) to 1 μg/ml} and collected RNA at 6 h and 24 h for gene expression analysis. To examine the effects on ER positive cells, we treated MCF7 cells with digitoxin at 1 μg/ml and collected RNA for RT-PCR analysis. In addition, we assayed the growth inhibitory effect of low doses of digitoxin combined with paclitaxel and determined combination index values.
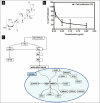
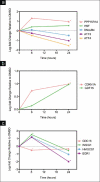
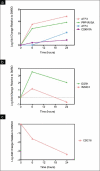
Results: To reveal primary effects, we examined digitoxin's effect 6 h post-treatment with the highest dose, 1μg/ml, and found upregulation of the stress response genes EGR-1 and NAB2, lipid biosynthetic genes and the tumor suppressor gene p21, and downregulation of the mitotic cell cycle gene CDC16 and the replication gene PolR3B. RT-PCR analysis validated effects on stress response, apoptotic and cell cycle genes on MDA-MB-453 and MCF7 cells. Western blot analysis confirmed induction of EGR1 protein at 1 h and ATF3 at 24 h. Paclitaxel, as well as digitoxin, inhibited the in vitro activity of the purified Na(+)-K(+)-ATPase; digitoxin enhanced the growth inhibitory effects of paclitaxel on Her2 overexpressing breast cancer cells.
Conclusions: Our studies show the potential of digitoxin to prevent and treat breast cancer and indicate that the combination of digitoxin and paclitaxel is a promising treatment for ER negative breast cancer. These findings are the first to alert physicians to the possible dangers to patients who take a combination of digitoxin and paclitaxel. The potential dangers ensuing when paclitaxel and digitoxin are combined are dependent on the dose of digitoxin.
Keywords: Cardiac glycosides; microarrays; paclitaxel; stress response; synergy.
Figures


Similar articles
-
Transcriptomic analysis of digitoxin: Synergy with doxorubicin in HER2-overexpressing MDA-MB-453 breast cancer cells.Biochimie. 2025 Jul;234:95-109. doi: 10.1016/j.biochi.2025.04.001. Epub 2025 Apr 4. Biochimie. 2025. PMID: 40188858
-
Digitoxin enhances the growth inhibitory effects of thapsigargin and simvastatin on ER negative human breast cancer cells.Fitoterapia. 2016 Mar;109:146-54. doi: 10.1016/j.fitote.2015.12.005. Epub 2015 Dec 12. Fitoterapia. 2016. PMID: 26691294
-
Actein inhibits the Na+-K+-ATPase and enhances the growth inhibitory effect of digitoxin on human breast cancer cells.Biochem Biophys Res Commun. 2008 Oct 31;375(4):608-13. doi: 10.1016/j.bbrc.2008.08.054. Epub 2008 Aug 26. Biochem Biophys Res Commun. 2008. PMID: 18755149
-
Carnosic acid inhibits the growth of ER-negative human breast cancer cells and synergizes with curcumin.Fitoterapia. 2012 Oct;83(7):1160-8. doi: 10.1016/j.fitote.2012.07.006. Epub 2012 Jul 22. Fitoterapia. 2012. PMID: 22828666
-
3,3'-diindolylmethane and paclitaxel act synergistically to promote apoptosis in HER2/Neu human breast cancer cells.J Surg Res. 2006 May 15;132(2):208-13. doi: 10.1016/j.jss.2006.02.008. Epub 2006 Mar 31. J Surg Res. 2006. PMID: 16580691
Cited by
-
Antitumor effects of naturally occurring cardiac glycosides convallatoxin and peruvoside on human ER+ and triple-negative breast cancers.Cell Death Discov. 2017 Feb 27;3:17009. doi: 10.1038/cddiscovery.2017.9. eCollection 2017. Cell Death Discov. 2017. PMID: 28250972 Free PMC article.
-
Activating Transcription Factor 3 as a Novel Regulator of Chemotherapy Response in Breast Cancer.Transl Oncol. 2018 Aug;11(4):988-998. doi: 10.1016/j.tranon.2018.06.001. Epub 2018 Jun 22. Transl Oncol. 2018. PMID: 29940414 Free PMC article.
-
Digitoxin and its synthetic analog MonoD have potent antiproliferative effects on lung cancer cells and potentiate the effects of hydroxyurea and paclitaxel.Oncol Rep. 2016 Feb;35(2):878-86. doi: 10.3892/or.2015.4416. Epub 2015 Nov 12. Oncol Rep. 2016. PMID: 26573786 Free PMC article.
-
HE4 promotes collateral resistance to cisplatin and paclitaxel in ovarian cancer cells.J Ovarian Res. 2016 May 17;9(1):28. doi: 10.1186/s13048-016-0240-0. J Ovarian Res. 2016. PMID: 27184254 Free PMC article.
-
Cardiac glycosides use and the risk and mortality of cancer; systematic review and meta-analysis of observational studies.PLoS One. 2017 Jun 7;12(6):e0178611. doi: 10.1371/journal.pone.0178611. eCollection 2017. PLoS One. 2017. PMID: 28591151 Free PMC article.
References
-
- Stenkvist B. Is digitalis a therapy for breast carcinoma? Oncol Rep. 1999;6:493–6. - PubMed
-
- Kometiani P, Liu L, Askari A. Digitalis-induced signaling by Na+/K+-ATPase in human breast cancer cells. Mol Pharmacol. 2005;67:929–36. - PubMed
-
- Lopez-Lazaro M, Palma De La Pena N, Pastor N, Martín-Cordero C, Navarro E, Cortés F, et al. Anti-tumour activity of digitalis purpurea L. subsp. heywoodii. Planta Med. 2003;69:701–4. - PubMed
-
- McConkey D, Lin Y, Nutt L, Ozel H, Newman R. Cardiac glycosides stimulate Ca2+ increases and apoptosis in androgen-independent, metastatic human prostate adenocarcinoma cells. Cancer Res. 2000;60:3807–12. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

