Tri-trophic effects of inter- and intra-population variation in defence chemistry of wild cabbage (Brassica oleracea)
- PMID: 21140168
- PMCID: PMC3094535
- DOI: 10.1007/s00442-010-1861-4
Tri-trophic effects of inter- and intra-population variation in defence chemistry of wild cabbage (Brassica oleracea)
Abstract
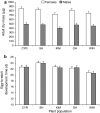
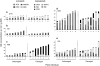
The effect of direct chemical defences in plants on the performance of insect herbivores and their natural enemies has received increasing attention over the past 10 years. However, much less is known about the scale at which this variation is generated and maintained, both within and across populations of the same plant species. This study compares growth and development of the large cabbage butterfly, Pieris brassicae, and its gregarious pupal parasitoid, Pteromalus puparum, on three wild populations [Kimmeridge (KIM), Old Harry (OH) and Winspit (WIN)] and two cultivars [Stonehead (ST), and Cyrus (CYR)] of cabbage, Brassica oleracea. The wild populations originate from the coast of Dorset, UK, but grow in close proximity with one another. Insect performance and chemical profiles were made from every plant used in the experiment. Foliar glucosinolates (GS) concentrations were highest in the wild plants in rank order WIN > OH > KIM, with lower levels found in the cultivars. Caterpillar-damaged leaves in the wild cabbages also had higher GS levels than undamaged leaves. Pupal mass in P. brassicae varied significantly among populations of B. oleracea. Moreover, development time in the host and parasitoid were correlated, even though these stages are temporally separated. Parasitoid adult dry mass closely approximated the development of its host. Multivariate statistics revealed a correlation between pupal mass and development time of P. brassicae and foliar GS chemistry, of which levels of neoglucobrassicin appeared to be the most important. Our results show that there is considerable variation in quantitative aspects of defensive chemistry in wild cabbage plants that is maintained at very small spatial scales in nature. Moreover, the performance of the herbivore and its parasitoid were both affected by differences in plant quality.
Figures


References
-
- Agrawal AA. Induced plant defense: evolution of induction and adaptive phenotypic plasticity. In: Agrawal AA, Tuzun S, Bent E, editors. Induced plant defenses against pathogens and herbivores: biochemistry, ecology and agriculture. Minosota: APS; 1999. pp. 251–268.
-
- Askew RR, Shaw MR. Parasitoid communities: their size, structure, and development. In: Waage J, Greathead D, editors. Insect parasitoids. London: Academic; 1986. pp. 225–264.
-
- Barbosa P, Saunders JA, Kemper J, Trumbule R, Olechno J, Martinat P. Plant allelochemicals and insect parasitoids: effects of nicotine on Cotesia congregata (Say) (Hymenoptera, Braconidae) and Hyposoter annulipes (Cresson) (Hymenoptera, Ichneumonidae) J Chem Ecol. 1986;12:1319–1328. doi: 10.1007/BF01012351. - DOI - PubMed
-
- Barbosa P, Gross P, Kemper J. Influence of plant allelochemicals on the tobacco hornworm and its parasitoid, Cotesia congregata. Ecology. 1991;72:1567–1575. doi: 10.2307/1940956. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

