Update 1 of: Tunneling and dynamics in enzymatic hydride transfer
- PMID: 21141912
- PMCID: PMC4067601
- DOI: 10.1021/cr1001035
Update 1 of: Tunneling and dynamics in enzymatic hydride transfer
Figures
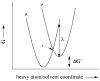
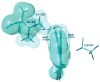
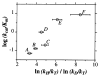
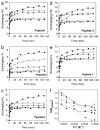
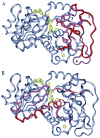
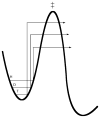
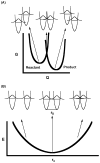

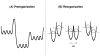
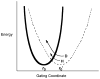


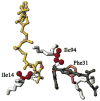


Republished from
-
Tunneling and dynamics in enzymatic hydride transfer.Chem Rev. 2006 Aug;106(8):3095-118. doi: 10.1021/cr050301x. Chem Rev. 2006. PMID: 16895320 Review. No abstract available.
References
Grants and funding
LinkOut - more resources
Full Text Sources

