Site-directed mutants of 16S rRNA reveal important RNA domains for KsgA function and 30S subunit assembly
- PMID: 21142019
- PMCID: PMC4355582
- DOI: 10.1021/bi101005r
Site-directed mutants of 16S rRNA reveal important RNA domains for KsgA function and 30S subunit assembly
Abstract
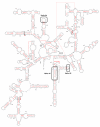
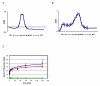
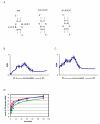
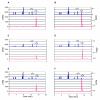
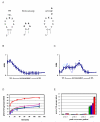
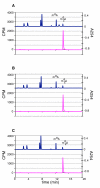
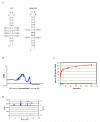
KsgA is an rRNA methyltransferase important to the process of small subunit biogenesis in bacteria. It is ubiquitously found in all life including archaea and eukarya, where the enzyme is referred to as Dim1. Despite the emergence of considerable data addressing KsgA function over the last several years, details pertaining to RNA recognition are limited, in part because the most accessible substrate for in vitro studies of KsgA is the 900000 Da 30S ribosomal subunit. To overcome challenges imposed by size and complexity, we adapted recently reported techniques to construct in vivo assembled mutant 30S subunits suitable for use in in vitro methyltransferase assays. Using this approach, numerous 16S rRNA mutants were constructed and tested. Our observations indicate that the 790 loop of helix 24 plays an important role in overall catalysis by KsgA. Moreover, the length of helix 45 also is important to catalysis. In both cases loss of catalytic function occurred without an increase in the production of N(6)-methyladenosine, a likely indication that there was no critical reduction in binding strength. Both sets of observations support a "proximity" mechanism of KsgA function. We also report that several of the mutants constructed failed to assemble properly into 30S subunits, while some others did so with reduced efficiency. Therefore, the same technique of generating mutant 30S subunits can be used to study ribosome biogenesis on the whole.
Figures
Similar articles
-
Structural insights into methyltransferase KsgA function in 30S ribosomal subunit biogenesis.J Biol Chem. 2012 Mar 23;287(13):10453-10459. doi: 10.1074/jbc.M111.318121. Epub 2012 Feb 3. J Biol Chem. 2012. PMID: 22308031 Free PMC article.
-
Mechanistic insight into the ribosome biogenesis functions of the ancient protein KsgA.Mol Microbiol. 2008 Dec;70(5):1062-75. doi: 10.1111/j.1365-2958.2008.06485.x. Mol Microbiol. 2008. PMID: 18990185 Free PMC article.
-
Modification of 16S ribosomal RNA by the KsgA methyltransferase restructures the 30S subunit to optimize ribosome function.RNA. 2010 Dec;16(12):2319-24. doi: 10.1261/rna.2357210. Epub 2010 Oct 20. RNA. 2010. PMID: 20962038 Free PMC article.
-
Structural insights into cell cycle control by essential GTPase Era.Postepy Biochem. 2016;62(3):335-342. Postepy Biochem. 2016. PMID: 28132488 Free PMC article. Review.
-
RNA-protein interactions in 30S ribosomal subunits: folding and function of 16S rRNA.Science. 1989 May 19;244(4906):783-90. doi: 10.1126/science.2658053. Science. 1989. PMID: 2658053 Review.
Cited by
-
Structural insights into methyltransferase KsgA function in 30S ribosomal subunit biogenesis.J Biol Chem. 2012 Mar 23;287(13):10453-10459. doi: 10.1074/jbc.M111.318121. Epub 2012 Feb 3. J Biol Chem. 2012. PMID: 22308031 Free PMC article.
-
Decoding the Mechanism of Specific RNA Targeting by Ribosomal Methyltransferases.ACS Chem Biol. 2022 Apr 15;17(4):829-839. doi: 10.1021/acschembio.1c00732. Epub 2022 Mar 22. ACS Chem Biol. 2022. PMID: 35316014 Free PMC article.
-
A single methyltransferase YefA (RlmCD) catalyses both m5U747 and m5U1939 modifications in Bacillus subtilis 23S rRNA.Nucleic Acids Res. 2011 Nov;39(21):9368-75. doi: 10.1093/nar/gkr626. Epub 2011 Aug 8. Nucleic Acids Res. 2011. PMID: 21824914 Free PMC article.
-
Survey and Validation of tRNA Modifications and Their Corresponding Genes in Bacillus subtilis sp Subtilis Strain 168.Biomolecules. 2020 Jun 30;10(7):977. doi: 10.3390/biom10070977. Biomolecules. 2020. PMID: 32629984 Free PMC article.
-
TRMT2B is responsible for both tRNA and rRNA m5U-methylation in human mitochondria.RNA Biol. 2020 Apr;17(4):451-462. doi: 10.1080/15476286.2020.1712544. Epub 2020 Jan 17. RNA Biol. 2020. PMID: 31948311 Free PMC article.
References
-
- Helser TL, Davies JE, Dahlberg JE. Change in Methylation of 16S Ribosomal RNA Associated with Mutation to Kasugamycin Resistance in Escherichia Coli. Nat. New Biol. 1971;233:12–14. - PubMed
-
- Poldermans B, Roza L, Van Knippenberg PH. Studies on the Function of Two Adjacent N6, N6-Dimethyladenosines Near the 3′ End of 16 S Ribosomal RNA of Escherichia Coli. III. Purification and Properties of the Methylating Enzyme and Methylase-30 S Interactions. J. Biol. Chem. 1979;254:9094–9100. - PubMed
-
- Van Buul CP, Damm JB, Van Knippenberg PH. Kasugamycin Resistant Mutants of Bacillus Stearothermophilus Lacking the Enzyme for the Methylation of Two Adjacent Adenosines in 16S Ribosomal RNA. Mol. Gen. Genet. 1983;189:475–478. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

