Fast photochemical oxidation of proteins for comparing structures of protein-ligand complexes: the calmodulin-peptide model system
- PMID: 21142124
- PMCID: PMC3078576
- DOI: 10.1021/ac102426d
Fast photochemical oxidation of proteins for comparing structures of protein-ligand complexes: the calmodulin-peptide model system
Abstract
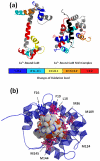
Fast photochemical oxidation of proteins (FPOP) is a mass spectrometry-based protein footprinting method that modifies proteins on the microsecond time scale. Highly reactive (•)OH, produced by laser photolysis of hydrogen peroxide, oxidatively modifies the side chains of approximately one-half the common amino acids on this time scale. Because of the short labeling exposure, only solvent-accessible residues are sampled. Quantification of the modification extent for the apo and holo states of a protein-ligand complex provides structurally sensitive information at the amino-acid level to compare the structures of unknown protein complexes with known ones. We report here the use of FPOP to monitor the structural changes of calmodulin in its established binding to M13 of the skeletal muscle myosin light chain kinase. We use the outcome to establish the unknown structures resulting from binding with melittin and mastoparan. The structural comparison follows a comprehensive examination of the extent of FPOP modifications as measured by proteolysis and LC-MS/MS for each protein-ligand equilibrium. The results not only show that the three calmodulin-peptide complexes have similar structures but also reveal those regions of the protein that became more or less solvent-accessible upon binding. This approach has the potential for relatively high throughput, information-dense characterization of a series of protein-ligand complexes in biochemistry and drug discovery when the structure of one reference complex is known, as is the case for calmodulin and M13 of the skeletal muscle myosin light chain kinase, and the structures of related complexes are not.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

