Asymmetric synthesis of tertiary benzylic alcohols
- PMID: 21142190
- PMCID: PMC3028934
- DOI: 10.1021/ol102567h
Asymmetric synthesis of tertiary benzylic alcohols
Abstract
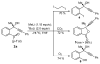
Vinyl, aryl, and alkynyl organometallics add to ketones containing a stereogenic sulfoxide. Tertiary alcohols are generated in diastereomerically and enantiomerically pure form. Reductive lithiation converts the sulfoxide into a variety of useful functional groups.
Figures
References
-
- Trost BM, Flemming I, editors. Comprehensive Organic Synthesis. 1-2. PERMAGON PRESS; Oxford: 1991.
-
-
Allylation: Denmark SE, Fu J. Chem. Rev. 2003;103:2763.
-
-
-
Arylation: Schmidt F, Stemmler RT, Rudolph J, Bolm C. Chem. Soc. Rev. 2006;35:454.
-
-
-
Vinylation: Oppolzer W, Radinov RN. Helv. Chim. Acta. 1992;75:170. Wipf P, Ribe S. J. Org. Chem. 1998;63:6454. Salvi L, Jeon S–J, Fisher EL, Carroll PJ, Walsh PJ. J. Am. Chem. Soc. 2007;129:16119.
-
-
-
Alkylation: Soai K, Niwa S. Chem. Rev. 1992;92:833. Pu L, Yu H-B. Chem. Rev. 2001;101:757. Muramatsu Y, Harada T. Angew. Chem., Int. Ed. 2008;47:1088.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources