Fabricating gradient hydrogel scaffolds for 3D cell culture
- PMID: 21143178
- PMCID: PMC3381353
- DOI: 10.2174/138620711795222455
Fabricating gradient hydrogel scaffolds for 3D cell culture
Abstract
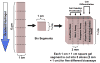
Optimizing cell-material interactions is critical for maximizing regeneration in tissue engineering. Combinatorial and high-throughput (CHT) methods can be used to systematically screen tissue scaffolds to identify optimal biomaterial properties. Previous CHT platforms in tissue engineering have involved a two-dimensional (2D) cell culture format where cells were cultured on material surfaces. However, these platforms are inadequate to predict cellular response in a three-dimensional (3D) tissue scaffold. We have developed a simple CHT platform to screen cell-material interactions in 3D culture format that can be applied to screen hydrogel scaffolds. Herein we provide detailed instructions on a method to prepare gradients in elastic modulus of photopolymerizable hydrogels.
Conflict of interest statement
The authors have no conflict of interests to declare.
Figures




References
-
- Lysaght MJ, Jaklenec A, Deweerd E. Great expectations: Private sector activity in tissue engineering, regenerative medicine, and stem cell therapeutics. Tissue Eng Part A. 2008;14(2):305–315. - PubMed
-
- Inglese J, Johnson RL, Simeonov A, Xia M, Zheng W, Austin CP, Auld DS. High-throughput screening assays for the identification of chemical probes. Nat Chem Biol. 2007;3(8):466–479. - PubMed
-
- Simon CG, Yang Y, Thomas V, Dorsey SM, Morgan AW. Cell interactions with biomaterials gradients and arrays. Comb Chem High Throughput Screen. 2009;12(6):544–553. - PubMed
-
- Meredith JC, Sormana JL, Keselowsky BG, Garcia AJ, Tona A, Karim A, Amis EJ. Combinatorial characterization of cell interactions with polymer surfaces. J Biomed Mater Res Part A. 2003;66(3):483–490. - PubMed
-
- Anderson DG, Levenberg S, Langer R. Nanoliter-scale synthesis of arrayed biomaterials and application to human embryonic stem cells. Nat Biotechnol. 2004;22(7):863–866. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

