Low-cost production of proinsulin in tobacco and lettuce chloroplasts for injectable or oral delivery of functional insulin and C-peptide
- PMID: 21143365
- PMCID: PMC3480330
- DOI: 10.1111/j.1467-7652.2010.00582.x
Low-cost production of proinsulin in tobacco and lettuce chloroplasts for injectable or oral delivery of functional insulin and C-peptide
Abstract
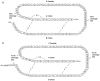
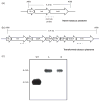
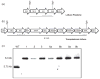
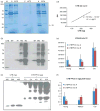
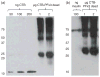
Current treatment for type I diabetes includes delivery of insulin via injection or pump, which is highly invasive and expensive. The production of chloroplast-derived proinsulin should reduce cost and facilitate oral delivery. Therefore, tobacco and lettuce chloroplasts were transformed with the cholera toxin B subunit fused with human proinsulin (A, B, C peptides) containing three furin cleavage sites (CTB-PFx3). Transplastomic lines were confirmed for site-specific integration of transgene and homoplasmy. Old tobacco leaves accumulated proinsulin up to 47% of total leaf protein (TLP). Old lettuce leaves accumulated proinsulin up to 53% TLP. Accumulation was so stable that up to ~40% proinsulin in TLP was observed even in senescent and dried lettuce leaves, facilitating their processing and storage in the field. Based on the yield of only monomers and dimers of proinsulin (3 mg/g leaf, a significant underestimation), with a 50% loss of protein during the purification process, one acre of tobacco could yield up to 20 million daily doses of insulin per year. Proinsulin from tobacco leaves was purified up to 98% using metal affinity chromatography without any His-tag. Furin protease cleaved insulin peptides in vitro. Oral delivery of unprocessed proinsulin bioencapsulated in plant cells or injectable delivery into mice showed reduction in blood glucose levels similar to processed commercial insulin. C-peptide should aid in long-term treatment of diabetic complications including stimulation of nerve and renal functions. Hyper-expression of functional proinsulin and exceptional stability in dehydrated leaves offer a low-cost platform for oral and injectable delivery of cleavable proinsulin.
© 2010 The Authors. Plant Biotechnology Journal © 2010 Society for Experimental Biology, Association of Applied Biologists and Blackwell Publishing Ltd.
Figures




References
-
- Agarwal V, Khan M. Current status of the oral delivery of insulin. Pharm Technol. 2001 Oct;:76–90.
-
- American Diabetes Association. Economic costs of diabetes in the U.S. in 2007. Diabetes Care. 2008;31:596–615. - PubMed
-
- Arakawa T, Yu J, Chong DK, Hough J, Engen PC, Langridge WH. A plant-based cholera toxin B subunit-insulin fusion protein protects against the development of autoimmune diabetes. Nat Biotechnol. 1998;16:934–938. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

