Cathepsins and their endogenous inhibitors cystatins: expression and modulation in multiple sclerosis
- PMID: 21143385
- PMCID: PMC3822953
- DOI: 10.1111/j.1582-4934.2010.01229.x
Cathepsins and their endogenous inhibitors cystatins: expression and modulation in multiple sclerosis
Abstract
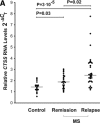
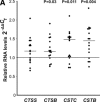
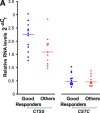
Cathepsins are involved in a variety of physiological processes including antigen processing and presentation and extracellular matrix degradation. In the present study, we evaluated whether expression levels of cathepsins S and B and their inhibitors cystatins B and C are affected by multiple sclerosis (MS) disease state (relapse and remission) and therapies (interferon-β [IFN-β] and the glucocorticoid [GC] methylprednisolone), and whether they are associated with the IFN-β response phenotype. Real-time PCR was employed to compare RNA expression levels in peripheral blood leucocytes (PBLs) and ELISA to determine serum protein levels of MS patients and matched healthy individuals. Cathepsin S RNA was higher in MS patients in the relapse state compared to controls (by 74%, P = 3 × 10(-5), n = 30 versus n = 18) with a similar increase observed in serum (66%, P = 0.002, n = 18 versus n = 20). GC treatment reduced cathepsin S levels in PBL RNA (by 44%, P = 6 × 10(-6), n = 27) and serum proteins (by 27%, P = 1 × 10(-5), n = 26), reduced the serum protein levels of pro-cathepsin B (by 8%, P = 0.0007, n = 23), and in parallel increased the serum levels of their inhibitor cystatin C (by 82%, P = 8 × 10(-6), n = 26). IFN-β therapy significantly elevated the RNA levels (n = 16) of cathepsin B (by 16%, P = 0.03), cystatin B (44%, P = 0.004) and cystatin C (48%, P = 0.011). In the serum, only cathepsin S levels were reduced by IFN-β (16%, P = 0.006, n = 25). Interestingly, pre-treatment serum cathepsin S/cystatin C ratio was higher in 'good responders' to IFN-β therapy compared to patients without a good response (by 94%, P = 0.003). These results suggest that cathepsin S and cystatin C may contribute to disease activity in MS, specifically in a subgroup of patients that are responsive to IFN-β therapy, and that these proteins should be further evaluated as biomarkers in MS.
© 2011 The Authors Journal of Cellular and Molecular Medicine © 2011 Foundation for Cellular and Molecular Medicine/Blackwell Publishing Ltd.
Figures









References
-
- Weiner HL. The challenge of multiple sclerosis: how do we cure a chronic heterogeneous disease. Ann Neurol. 2009;65:239–48. - PubMed
-
- Compston A, Coles A. Multiple sclerosis. Lancet. 2008;372:1502–17. - PubMed
-
- Chandler S, Miller KM, Clements JM, et al. Matrix metalloproteinases, tumor necrosis factor and multiple sclerosis: an overview. J Neuroimmunol. 1997;72:155–61. - PubMed
-
- Agrawal SM, Lau L, Yong VW. MMPs in the central nervous system: where the good guys go bad. Semin Cell Dev Biol. 2008;19:42–51. - PubMed
-
- Galboiz Y, Shapiro S, Lahat N, et al. Matrix metalloproteinases and their tissue inhibitors as markers of disease subtype and response to interferon-beta therapy in relapsing and secondary-progressive multiple sclerosis patients. Ann Neurol. 2001;50:443–51. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

