Post-translational inactivation of endothelial nitric oxide synthase in the transgenic sickle cell mouse penis
- PMID: 21143412
- PMCID: PMC5497993
- DOI: 10.1111/j.1743-6109.2010.02123.x
Post-translational inactivation of endothelial nitric oxide synthase in the transgenic sickle cell mouse penis
Abstract
Introduction: Sickle cell disease (SCD)-associated priapism is characterized by endothelial nitric oxide synthase (eNOS) dysfunction in the penis. However, the mechanism of decreased eNOS function/activation in the penis in association with SCD is not known.
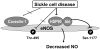
Aims: Our hypothesis in the present study was that eNOS is functionally inactivated in the SCD penis in association with impairments in eNOS post-translational phosphorylation and the enzyme's interactions with its regulatory proteins.
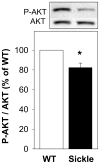
Methods: Sickle cell transgenic (sickle) mice were used as an animal model of SCD. Wild-type (WT) mice served as controls. Penes were excised at baseline for molecular studies. eNOS phosphorylation on Ser-1177 (positive regulatory site) and Thr-495 (negative regulatory site), total eNOS, and phosphorylated AKT (upstream mediator of eNOS phosphorylation on Ser-1177) expressions, and eNOS interactions with heat-shock protein 90 (HSP90) and caveolin-1 were measured by Western blot. Constitutive NOS catalytic activity was measured by conversion of L-[14C]arginine-to-L-[14C]citrulline in the presence of calcium.
Main outcome measures: Molecular mechanisms of eNOS dysfunction in the sickle mouse penis.
Results: eNOS phosphorylated on Ser-1177, an active portion of eNOS, was decreased in the sickle mouse penis compared with WT penis. eNOS interaction with its positive protein regulator HSP90, but not with its negative protein regulator caveolin-1, and phosphorylated AKT expression, as well as constitutive NOS activity, were also decreased in the sickle mouse penis compared with WT penis. eNOS phosphorylated on Thr-495, total eNOS, HSP90, and caveolin-1 protein expressions in the penis were not affected by SCD.
Conclusions: These findings provide a molecular basis for chronically reduced eNOS function in the penis by SCD, which involves decreased eNOS phosphorylation on Ser-1177 and decreased eNOS-HSP90 interaction.
© 2010 International Society for Sexual Medicine.
Figures



Similar articles
-
Testosterone replacement in transgenic sickle cell mice controls priapic activity and upregulates PDE5 expression and eNOS activity in the penis.Andrology. 2018 Jan;6(1):184-191. doi: 10.1111/andr.12442. Epub 2017 Nov 16. Andrology. 2018. PMID: 29145710 Free PMC article.
-
Sildenafil promotes eNOS activation and inhibits NADPH oxidase in the transgenic sickle cell mouse penis.J Sex Med. 2014 Feb;11(2):424-30. doi: 10.1111/jsm.12391. Epub 2013 Nov 20. J Sex Med. 2014. PMID: 24251665 Free PMC article.
-
Post-translational regulation of endothelial nitric oxide synthase (eNOS) by estrogens in the rat vagina.J Sex Med. 2010 May;7(5):1768-77. doi: 10.1111/j.1743-6109.2010.01750.x. Epub 2010 Mar 11. J Sex Med. 2010. PMID: 20233295 Free PMC article.
-
Posttranslational modification of constitutive nitric oxide synthase in the penis.J Androl. 2009 Jul-Aug;30(4):352-62. doi: 10.2164/jandrol.108.006999. Epub 2009 Apr 2. J Androl. 2009. PMID: 19342700 Free PMC article. Review.
-
eNOS function and dysfunction in the penis.Exp Biol Med (Maywood). 2006 Feb;231(2):154-65. doi: 10.1177/153537020623100205. Exp Biol Med (Maywood). 2006. PMID: 16446491 Review.
Cited by
-
Gas what: NO is not the only answer to sexual function.Br J Pharmacol. 2015 Mar;172(6):1434-54. doi: 10.1111/bph.12700. Epub 2014 Jul 2. Br J Pharmacol. 2015. PMID: 24661203 Free PMC article. Review.
-
The endocannabinoid system's genetic polymorphisms in sickle cell anemia patients.Sci Rep. 2024 Dec 30;14(1):31562. doi: 10.1038/s41598-024-76480-0. Sci Rep. 2024. PMID: 39738165 Free PMC article.
-
Targeting NADPH oxidase decreases oxidative stress in the transgenic sickle cell mouse penis.J Sex Med. 2012 Aug;9(8):1980-7. doi: 10.1111/j.1743-6109.2012.02798.x. Epub 2012 May 23. J Sex Med. 2012. PMID: 22620981 Free PMC article.
-
Testosterone replacement in transgenic sickle cell mice controls priapic activity and upregulates PDE5 expression and eNOS activity in the penis.Andrology. 2018 Jan;6(1):184-191. doi: 10.1111/andr.12442. Epub 2017 Nov 16. Andrology. 2018. PMID: 29145710 Free PMC article.
-
Hydroxyurea does not reverse functional alterations of the nitric oxide-cGMP pathway associated with priapism phenotype in corpus cavernosum from sickle cell mouse.PLoS One. 2023 Oct 9;18(10):e0292706. doi: 10.1371/journal.pone.0292706. eCollection 2023. PLoS One. 2023. PMID: 37812620 Free PMC article.
References
-
- Wood KC, Granger DN. Sickle cell disease: role of reactive oxygen and nitrogen metabolites. Clin Exp Pharmacol Physiol. 2007;34:926–932. - PubMed
-
- Montague DK, Jarow J, Broderick GA, Dmochowski RR, Heaton JP, Lue TF, Nehra A, Sharlip ID Members of the Erectile Dysfunction Guideline Update Panel, Americal Urological Association. American Urological Association Guideline on the management of priapism. J Urol. 2003;170:1318–1324. - PubMed
-
- Broderick GA, Kadioglu A, Bivalacqua TJ, Ghanem H, Nehra A, Shamloul R. Priapism: pathogenesis, epidemiology, and management. J Sex Med. 2010;7:476–500. - PubMed

