Tobramycin disposition in ICU patients receiving a once daily regimen: population approach and dosage simulations
- PMID: 21143502
- PMCID: PMC3018027
- DOI: 10.1111/j.1365-2125.2010.03793.x
Tobramycin disposition in ICU patients receiving a once daily regimen: population approach and dosage simulations
Abstract
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT? It is well known that tobramycin given as an once daily dose according to the usual recommendations needs therapeutic drug monitoring by measurement of peak and trough concentrations. In the literature, there are only few published studies on the population pharmacokinetics of once daily tobramycin in critically ill patients. Glomerular filtration rate and bodyweight were identified as covariates contributing to the inter-individual variability in the disposition of aminoglycosides. The study, by Peris-Marti et al. [24], only evaluated the pharmacodynamic effectiveness of a 4 mg kg(-1) dose of tobramycin given once daily in critically ill patients. The authors concluded with a simulation showing that for a theoretical MIC of 1 or 2 mg l(-1) , a 7 mg kg(-1) dose was required.
What this study adds: Our results confirm the high variability of tobramycin disposition in intensive care patients and consequently the possible lack of effectiveness. By using a population pharmacokinetic approach, two explicative covariates (height and Cockcroft creatinine clearance) added to a two-compartment model with proportional error, explained much of the inter-individual variability of tobramycin disposition in the critically ill patient population. In a median ICU patient, simulations were performed at various dosage regimens and peak and AUC pharmacodynamic targets could not be reached simultaneously in more than 45% of the ICU patient population. Drug monitoring is required to manage efficacy and toxicity.
Aim: The aim of this study was to evaluate the disposition of tobramycin (TOB) in critically ill patients (ICU) by a population pharmacokinetic approach, to determine the covariates involved, and to simulate tobramycin dosage regimens.
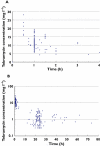
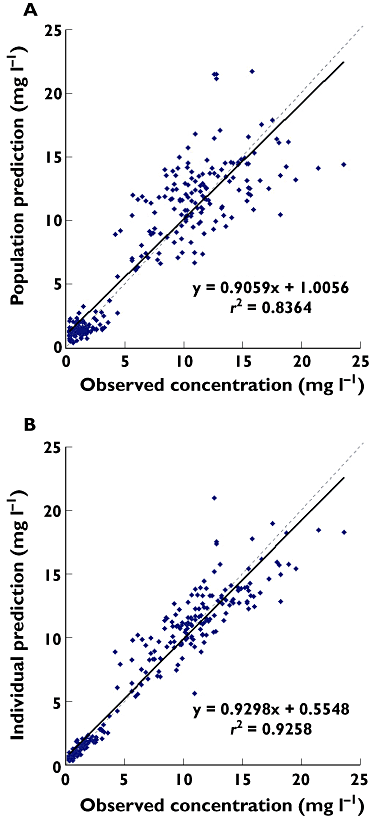
Methods: Forty-nine adult ICU patients received TOB (5 mg kg(-1) ) once daily. NonMem modelling was performed on 32 patients. The 17 other patients were used for the qualification process by normalized prediction distribution error. Then Monte Carlo simulations (MCS) were performed.
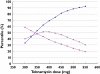
Results: A two-compartment model with a proportional error best fitted the data. TOB total clearance (CL(TOB) ) was significantly correlated with Cockcroft creatinine clearance (COCK) and height. TOB clearance was 4.8 ± 1.9 l h(-1) (range 1.22-8.95), the volume of distribution of the central compartment was 24.7 ± 3.7 l (range 17.34-32.83) and that of the peripheral compartment and the inter-compartmental clearance were 30.6 l and 4.74 l h(-1) , respectively. Only 29% of the patients presented a target AUC between 80 and 125 mg l(-1) h and 61% were lower than 80 mg l(-1) h. After considering COCK and height, MCS showed that only 50% of the population could achieve the target AUC for the 375 and 400 mg dosages.
Conclusion: Even after taking into account COCK and height, for strains with an MIC ≤ 1 mg l(-1) , MCS doses evidenced that peak and AUC pharmacodynamic targets could not be reached simultaneously in more than 45% of the ICU patient population. Combination therapy in addition to drug monitoring are required to manage efficacy and toxicity.
© 2010 The Authors. British Journal of Clinical Pharmacology © 2010 The British Pharmacological Society.
Figures
 ); Trough at 24 h <1 mg L−1 (
); Trough at 24 h <1 mg L−1 ( ); 80 < AUC < 125 mg L−1 h (
); 80 < AUC < 125 mg L−1 h ( )
)References
-
- Craig WA, Redington J, Ebert SC. Pharmacodynamics of amikacin in vitro and in mouse thigh and lung infections. J Antimicrob Chemother. 1991;27(Suppl. C):29–40. - PubMed
-
- Marik PE, Lipman J, Kobilski S, Scribante J. A prospective randomized study comparing once- versus twice-daily amikacin dosing in critically ill adult and paediatric patients. J Antimicrob Chemother. 1991;28:753–64. - PubMed
-
- Craig WA. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin Infect Dis. 1998;26:1–10. quiz 11–2. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

