IFN-γ response on T-cell based assays in HIV-infected patients for detection of tuberculosis infection
- PMID: 21143955
- PMCID: PMC3016378
- DOI: 10.1186/1471-2334-10-348
IFN-γ response on T-cell based assays in HIV-infected patients for detection of tuberculosis infection
Abstract
Background: Individuals infected with human immunodeficiency virus (HIV) have an increased risk of progression to active tuberculosis following Mycobacterium tuberculosis infection. The objective of the study was to determine IFN-γ responses for the detection of latent tuberculosis infection (LTBI) with QuantiFERON-TB GOLD In Tube (QFT-G-IT) and T-SPOT.TB in HIV patients, and evaluate the influence of CD4 cell count on tests performance.
Methods: We studied 75 HIV patients enrolled for ongoing studies of LTBI with T-SPOT.TB, QFN-G-IT and TST. Mean CD4 cell counts ± standard deviation was 461.29 ± 307.49 cells/μl. Eight patients had a BCG scar.
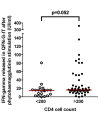
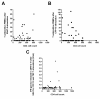
Results: T-SPOT.TB, QFN-G-IT and TST were positive in 7 (9.3%), 5 (6.7%) and 9 (12%) cases, respectively. Global agreement between QFN-G-IT and T-SPOT.TB was 89% (κ = 0.275). The overall agreement of T-SPOT.TB and QFN-G-IT with TST was 80.8% (κ = 0.019) and 89% (κ = 0.373), respectively. We have found negative IFN-γ assays results among 2 BCG-vaccinated HIV-infected individuals with a positive TST. In non BCG-vaccinated patients, QFN-G-IT and TST were positive in 5 cases (7.5%) and T-SPOT.TB in 7 (10.4%). In contrast, in BCG-vaccinated patients, only TST was positive in 4/8 (50%) of the cases. The differences obtained in the number of positive results between TST and both IFN-γ assays in BCG vaccinated patients were significant (95% CI 3-97%, p = 0.046), however, the confidence interval is very wide given the small number of patients. In patients with CD4< 200, we obtained only one (5%) positive result with T-SPOT.TB; however, QFN-G-IT and TST were negative in all cases. On the contrary, percentages of positive results in patients with CD4> 200 were 10.9% (6/55), 9.1% (5/55) and 16.4% (9/55) with T-SPOT.TB, QFN-G-IT and TST, respectively.
Conclusions: IFN-γ tests have the benefit over TST that are less influenced by BCG vaccination, consequently they are more specific than TST. Although our number of patients with advance immunosuppression is limited, our study suggests that IFN-γ assays are influenced with level of immunosuppression. The use of IFN-γ assays could be a helpful method for diagnosing LTBI in HIV population.
Figures
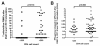
References
-
- World Health Organization. Global Tuberculosis Control - Surveillance, Planning, Financing (WHO/HTN/TB/2005.349). Geneva, Switzerland. 2005.
-
- Corbett EL, Charalambous S, Moloi VM, Fielding K, Grant AD, Dye C, De Cock KM, Hayes RJ, Williams BG, Churchyard GJ. Human immunodeficiency virus and the prevalence of undiagnosed tuberculosis in African gold miners. Am J Respir Crit Care Med. 2004;170:673–679. doi: 10.1164/rccm.200405-590OC. - DOI - PubMed
-
- Huebner RE, Schein MF, Bass JB Jr. The tuberculin skin test. Clin Infect Dis. 1993;17:968–975. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

