Examining and analyzing subcellular morphology of renal tissue treated by histotripsy
- PMID: 21144960
- PMCID: PMC3038584
- DOI: 10.1016/j.ultrasmedbio.2010.10.002
Examining and analyzing subcellular morphology of renal tissue treated by histotripsy
Abstract
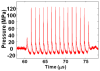
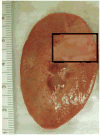
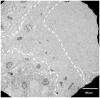
Our recent studies have shown that high-intensity pulsed ultrasound can achieve mechanical tissue fragmentation, a process we call histotripsy. Histotripsy has many medical applications where noninvasive tissue removal or significant tissue disruption is needed (e.g., cancer therapy). The primary aim of this study is to investigate tissue regions treated by histotripsy and to characterize the boundary between the treated and untreated zones using transmission electron microscopy (TEM). The nature of the tissue disruption suggests many clinical applications and provides insights on the physical mechanism of histotripsy. Fresh ex vivo porcine kidney tissues were treated using histotripsy. A 1 MHz 100 mm diameter focused transducer was used to deliver 15 cycle histotripsy pulses at a peak negative pressure of 17 MPa and a pulse repetition frequency (PRF) of 100 Hz. Each lesion was produced by a 3 × 3 (lateral) × 4 (axial) grid with 2 mm between adjacent lateral and 3 mm between axial exposure points using mechanical scanning. Two thousand pulses were applied to each exposure point to achieve tissue fragmentation. After treatment, the tissue was processed and examined using TEM. Extensive fragmentation of the tissues treated with histotripsy was achieved. TEM micrographs of the tissue treated by histotripsy, showing no recognizable cellular features and little recognizable subcellular structures, demonstrates the efficacy of this technique in ablating the targeted tissue regions. A boundary, or transition zone, of a few microns separated the affected and unaffected areas, demonstrating the precision of histotripsy tissue targeting. TEM micrographs of the tissue treated by histotripsy showed no discernable cellular structure within the treated region. Histotripsy can minimize fragmentation of the adjoining nontargeted tissues because, as a nonlinear threshold phenomenon, damage can be highly localized. The potential for high lesion precision is evident in the TEM micrographs.
Copyright © 2011 World Federation for Ultrasound in Medicine & Biology. Published by Elsevier Inc. All rights reserved.
Figures








References
-
- Anderson CD, Lin W-C, Beckham J, Mahadevan-Jansen A, Buttemere CR, Pierce J, Nicoud IB, Pinson CW, Chari RS. Fluorescence Spectroscopy Accurately Detects Irreversible Cell Damage During Hepatic Radiofrequency Ablation. Surgery. 2004 Sep;136(3):524–31. - PubMed
-
- Back W, Kohrmann KU, Bensemann J, Rassweiler J, Alken P. Histomorphologic and Ultrastructural Findings of Shockwave-Induced Lesions in the Isolated Perfused Kidney of the Pig. J Endourol. 1994;8(4):257–261. - PubMed
-
- Crosier AE, Farin PW, Dykstra MJ, Alexander JE, Farin C. Ultrastructural Morphometry of Bovine Blastocysts Produced In Vivo or In Vitro. Biol Reprod. 2001;64:1375–1385. - PubMed
-
- Glamoclija V, Vilović K, aga-Babić M, Baranović A, Sapunar D. Apoptosis and Active Caspase-3 Expression in Human Granulosa Cells. Fertil Steril. 2005;83:426–31. - PubMed
-
- Hsu THS, Fidler ME, Gill IS. Radiofrequency Ablation of the Kidney: Acute and Chronic Histology in Porcine Model. Urol. 2000;56:872–875. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

