Neural correlates of variations in event processing during learning in central nucleus of amygdala
- PMID: 21145010
- PMCID: PMC3033562
- DOI: 10.1016/j.neuron.2010.11.019
Neural correlates of variations in event processing during learning in central nucleus of amygdala
Abstract
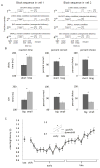
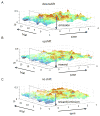
Attention or variations in event processing help drive learning. Lesion studies have implicated the central nucleus of the amygdala (CeA) in this process, particularly when expected rewards are omitted. However, lesion studies cannot specify how information processing in CeA supports such learning. To address these questions, we recorded CeA neurons in rats performing a task in which rewards were delivered or omitted unexpectedly. We found that activity in CeA neurons increased selectively at the time of omission and declined again with learning. Increased firing correlated with CeA-inactivation sensitive measures of attention. Notably CeA neurons did not fire to the cues or in response to unexpected rewards. These results indicate that CeA contributes to learning in response to reward omission due to a specific role in signaling actual omission rather than a more general involvement in signaling expectancies, errors, or reward value.
Copyright © 2010 Elsevier Inc. All rights reserved.
Figures

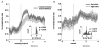
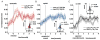

References
-
- Bucci DJ, Macleod JE. Changes in neural activity associated with a surprising change in the predictive validity of a conditioned stimulus. Eur J Neurosci. 2007;26:2669–2676. - PubMed
-
- Han JS, Holland PC, Gallagher M. Disconnection of the amygdala central nucleus and substantia innominata/nucleus basalis disrupts increments in conditioned stimulus processing in rats. Behav Neurosci. 1999;113:143–151. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

