Cisplatin induced mitochondrial DNA damage in dorsal root ganglion neurons
- PMID: 21145397
- PMCID: PMC3031677
- DOI: 10.1016/j.nbd.2010.11.017
Cisplatin induced mitochondrial DNA damage in dorsal root ganglion neurons
Abstract
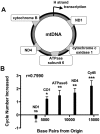
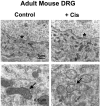
Cisplatin is a platinum-based chemotherapeutic agent that induces peripheral neuropathy in 30% of patients. Peripheral neuropathy is the dose limiting side effect, which has no preventative therapy. We have previously shown that cisplatin induces apoptosis in dorsal root ganglion (DRG) sensory neurons by covalently binding to nuclear DNA (nDNA), resulting in DNA damage, subsequent p53 activation and Bax-mediated apoptosis via the mitochondria. We now demonstrate that cisplatin also directly binds to mitochondrial DNA (mtDNA) with the same binding affinity as nDNA. Cisplatin binds 1 platinum molecule per 2166 mtDNA base pairs and 1 platinum molecule per 3800 nDNA base pairs. Furthermore, cisplatin treatment inhibits mtDNA replication as detected by 5-bromo-2'-deoxy-uridine (BrdU) incorporation and inhibits transcription of mitochondrial genes. The relative reduction in mtDNA transcription is directly related to the distance the gene is located from the transcription initiation point, which implies that randomly formed platinum adducts block transcription. Cisplatin treated DRG neurons exhibit mitochondrial vacuolization and degradation in vitro and in vivo. Taken together, this data suggests that direct mtDNA damage may provide a novel, distinct mechanism for cisplatin-induced neurotoxicity separate from the established nDNA damage pathway.
Copyright © 2010 Elsevier Inc. All rights reserved.
Figures




References
-
- Asin-Cayuela J, Gustafsson CM. Mitochondrial transcription and its regulation in mammalian cells. Trends Biochem Sci. 2007;32:111–7. - PubMed
-
- Cavaletti G. Peripheral neurotoxicity of platinum-based chemotherapy. Nat Rev Cancer. 2008;8 1p following 71; author reply 1p following 71. - PubMed
-
- Clayton DA. Replication and transcription of vertebrate mitochondrial DNA. Annu Rev Cell Biol. 1991;7:453–78. - PubMed
-
- Conti AM, et al. Inhibition of axonal growth from sensory neurons by excess nerve growth factor. Ann Neurol. 1997;42:838–846. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

