mTORC2 regulates neutrophil chemotaxis in a cAMP- and RhoA-dependent fashion
- PMID: 21145500
- PMCID: PMC3071587
- DOI: 10.1016/j.devcel.2010.11.004
mTORC2 regulates neutrophil chemotaxis in a cAMP- and RhoA-dependent fashion
Abstract
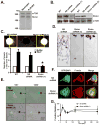
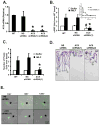
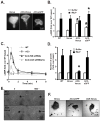
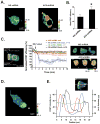
We studied the role of the target of rapamycin complex 2 (mTORC2) during neutrophil chemotaxis, a process that is mediated through the polarization of actin and myosin filament networks. We show that inhibition of mTORC2 activity, achieved via knock down (KD) of Rictor, severely inhibits neutrophil polarization and directed migration induced by chemoattractants, independently of Akt. Rictor KD also abolishes the ability of chemoattractants to induce cAMP production, a process mediated through the activation of the adenylyl cyclase 9 (AC9). Cells with either reduced or higher AC9 levels also exhibit specific and severe tail retraction defects that are mediated through RhoA. We further show that cAMP is excluded from extending pseudopods and remains restricted to the cell body of migrating neutrophils. We propose that the mTORC2-dependent regulation of MyoII occurs through a cAMP/RhoA-signaling axis, independently of actin reorganization during neutrophil chemotaxis.
Copyright © 2010 Elsevier Inc. All rights reserved.
Figures

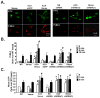
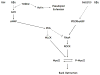
Comment in
-
"TORCing" neutrophil chemotaxis.Dev Cell. 2010 Dec 14;19(6):795-6. doi: 10.1016/j.devcel.2010.11.017. Dev Cell. 2010. PMID: 21145496 Free PMC article.
-
Cell migration: MTORC2 brings up the rear.Nat Rev Mol Cell Biol. 2011 Feb;12(2):74. doi: 10.1038/nrm3046. Epub 2011 Jan 12. Nat Rev Mol Cell Biol. 2011. PMID: 21224887 No abstract available.
References
-
- Alessi DR, Deak M, Casamayor A, Caudwell FB, Morrice N, Norman DG, Gaffney P, Reese CB, MacDougall CN, Harbison D, et al. 3-Phosphoinositide-dependent protein kinase-1 (PDK1): structural and functional homology with the Drosophila DSTPK61 kinase. Curr Biol. 1997;7:776–789. - PubMed
-
- Ali H, Sozzani S, Fisher I, Barr AJ, Richardson RM, Haribabu B, Snyderman R. Differential regulation of formyl peptide and platelet-activating factor receptors. Role of phospholipase Cbeta3 phosphorylation by protein kinase A. J Biol Chem. 1998;273:11012–11016. - PubMed
-
- Amano M, Ito M, Kimura K, Fukata Y, Chihara K, Nakano T, Matsuura Y, Kaibuchi K. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase) J Biol Chem. 1996;271:20246–20249. - PubMed
-
- Bagorda A, Mihaylov VA, Parent CA. Chemotaxis: moving forward and holding on to the past. Thromb Haemost. 2006;95:12–21. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

