Toxicity and tolerance mechanisms for azidothymidine, a replication gap-promoting agent, in Escherichia coli
- PMID: 21145792
- PMCID: PMC3046245
- DOI: 10.1016/j.dnarep.2010.11.007
Toxicity and tolerance mechanisms for azidothymidine, a replication gap-promoting agent, in Escherichia coli
Abstract
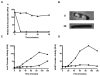
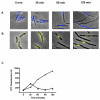
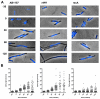
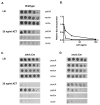
Azidothymidine (AZT, zidovudine) is used to treat HIV-AIDS and prevent maternal transmission to newborns. Because the azido group replaces the 3' OH of thymidine, AZT is believed to act as a chain terminator during reverse transcription of viral RNA into DNA, although other mechanisms of viral inhibition have been suggested. There is evidence that AZT is genotoxic, particularly to the mitochondria. In this study, we use the bacterium Escherichia coli to investigate the mechanism of AZT toxicity and the cellular mechanisms that aid survival. We show that that replication arrests quickly after treatment, accompanied by induction of the SOS DNA damage response. AZT appears to produce single-strand DNA gaps, as evident by RecF-dependent induction of the SOS response and visualization of single-strand DNA binding protein foci within the cell. Some of these gaps must be converted to breaks, since mutants in the RecBCD nuclease, required for recombinational double-strand break repair, are highly sensitive to AZT. Blocks in the late recombination functions, the RuvAB branch migration helicase and RuvC Holliday junction endonuclease, caused extreme AZT sensitivity that could be relieved by mutations in the early recombination functions, such as RecF, suggesting gaps engage in recombination reactions. Finally, our data suggest that the proofreading exonucleases of DNA polymerases play little role in AZT tolerance. Rather, Exonuclease III appears to be the enzyme that removes AZT: xthA mutants are highly AZT-sensitive, with a sustained SOS response, and overproduction of the enzyme protects wild-type cells. Our findings suggest that incorporation of AZT into human nuclear and mitochondrial DNA has the potential to promote genetic instability and toxicity through the production of ssDNA gaps and dsDNA breaks, and predicts that the human Exonuclease III ortholog, APE1, will be important for drug tolerance.
Copyright © 2010 Elsevier B.V. All rights reserved.
Figures



References
-
- Olivero OA. Mechanisms of genotoxicity of nucleoside reverse transcriptase inhibitors. Environ Mol Mutagen. 2007;48:215–223. - PubMed
-
- International Agency for Research on Cancer IARC monographs on the evaluation of carcinogenic risks to humans. Some antiviral and antineoplastic drugs and other pharmaceutical agents. 2000. pp. 1–521. (IARC Monographs).
-
- Kang D, Hamasaki N. Alterations of mitochondrial DNA in common diseases and disease states: aging, neurodegeneration, heart failure, diabetes, and cancer. Curr Med Chem. 2005;12:429–441. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

