Sarcopenia associated with portosystemic shunting is reversed by follistatin
- PMID: 21145817
- PMCID: PMC3118576
- DOI: 10.1016/j.jhep.2010.08.032
Sarcopenia associated with portosystemic shunting is reversed by follistatin
Abstract
Background & aims: The distinct role of portosystemic shunting (PSS) in the pathogenesis of sarcopenia (skeletal muscle loss) that occurs commonly in cirrhosis is unclear. We have previously shown increased expression of myostatin (inhibitor of skeletal muscle mass) in the portacaval anastamosis (PCA) rat model of sarcopenia of PSS. The present study was performed to examine the mechanisms of sarcopenia following PCA.
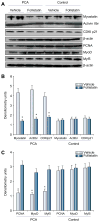
Methods: In PCA and sham operated pair fed control rats, the phenylalanine flooding dose method was used to quantify the fractional and absolute protein synthesis rates in the skeletal muscle over time and in response to follistatin, a myostatin antagonist. The expression of myostatin and markers of satellite cell (myocyte precursors) proliferation and differentiation were quantified by real-time PCR and Western blot analyses.
Results: The absolute synthesis rate (ASR) was lower at 2, 4, and 6 weeks (p<0.05) and the fractional synthesis rate (FSR) of skeletal muscle protein was significantly lower (p<0.05) at week 2 in the PCA rats compared to control rats. Expression of myostatin was elevated while markers of satellite cell proliferation and differentiation were lower at 4 and 6 weeks after PCA. Follistatin increased skeletal muscle mass, muscle FSR and ASR, decreased expression of myostatin protein, and increased expression of markers of satellite cell function.
Conclusions: Sarcopenia associated with PSS is caused by impaired protein synthesis and reduced satellite cell function due to increased myostatin expression. Confirming these alterations in human patients with cirrhosis will provide novel therapeutic targets for sarcopenia of liver disease.
Copyright © 2010 European Association for the Study of the Liver. Published by Elsevier B.V. All rights reserved.
Figures




References
-
- Selberg O, Bottcher J, Tusch G, Pichlmayr R, Henkel E, Muller MJ. Identification of high- and low-risk patients before liver transplantation: a prospective cohort study of nutritional and metabolic parameters in 150 patients. Hepatology. 1997;25 (3):652–657. - PubMed
-
- Dasarathy S, Mullen KD, Conjeevaram HS, Kaminsky-Russ K, Wills LA, McCullough AJ. Preservation of portal pressure improves growth and metabolic profile in the male portacaval-shunted rat. Dig Dis Sci. 2002;47 (9):1936–1942. - PubMed
-
- Kuang S, Rudnicki MA. The emerging biology of satellite cells and their therapeutic potential. Trends Mol Med. 2008;14 (2):82–91. - PubMed
-
- Lundborg H, Hamberger A. Effects of portacaval anastomosis on liver and brain protein synthesis in rats. Surgery. 1977;82 (5):643–647. - PubMed
-
- Dunlop DS, Kaufman H, Zanchin G, Lajtha A. Protein synthesis rates in rats with portacaval shunts. J Neurochem. 1984;43 (5):1487–1489. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

